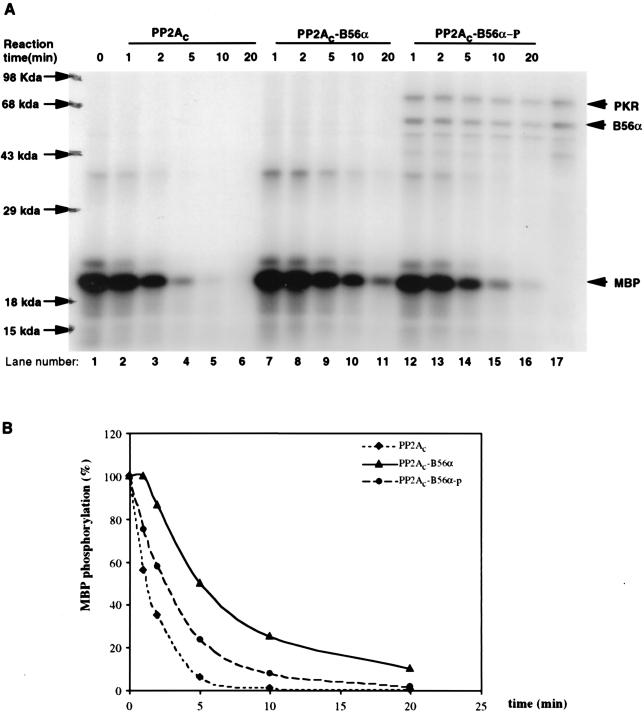
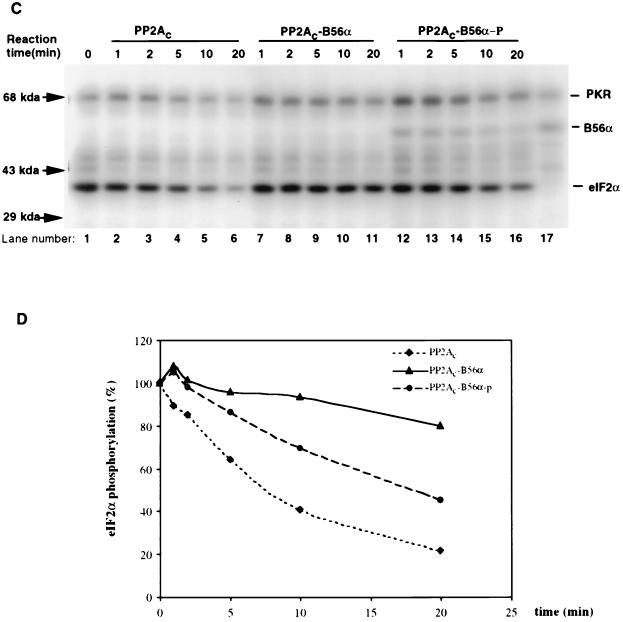
FIG. 5.
Modulation of PP2A activity on MBP or eIF2α via phosphorylation of B56α by PKR. (A) In vitro PP2A activity on PKC-phosphorylated MBP. MBP was phosphorylated by PKCα with radioactive ATP ([γ-32P]ATP) and used as a substrate for PP2A (either PP2Ac dimer, PP2Ac-B56α, or phosphorylated PP2Ac-B56α). B56α either phosphorylated or not by PKR was quickly premixed with PP2Ac to generate the PP2Ac-B56α or PP2Ac-B56α-p form of PP2A. Dephosphorylation was carried out at 30°C, and equal amounts of reaction mixture were taken out after 1, 2, 5, 10, and 20 min. The reaction was stopped by adding phosphatase stop solution and separated by SDS–12% PAGE. Lane 1, starting level of MBP phosphorylation; lanes 2 to 16, time courses of dephosphorylation of MBP; lane 17, input of PKR and B56α in PP2Ac-B56α-p reaction. (B) Quantitation of the data in panel A. MBP phosphorylation levels were quantitated by ImageQuant version 1.1 analysis following scanning of the gel with a Storm PhosphorImager. (C) In vitro PP2A activity on PKR-phosphorylated eIF2α. Recombinant eIF2α was phosphorylated by recombinant PKR with radioactive ATP ([γ-32P]ATP) and used as a substrate for PP2A (either AC dimer PP2Ac, PP2Ac-B56α, or PP2Ac-B56α-p). Dephosphorylation of eIF2α was carried out in the same way as for MBP dephosphorylation. Lane 1, starting level of eIF2α phosphorylation; lanes 2 to 6, time course of dephosphorylation of eIF2α by PP2Ac core dimer; lanes 7 to 11, time course of dephosphorylation of eIF2α by PP2Ac-B56α; lanes 12 to 16, time course of dephosphorylation of eIF2α by PP2Ac-B56α-p; lane 17, Input of PKR and B56α in PP2Ac-B56α-p reaction. (D) eIF2α phosphorylation levels were quantitated by ImageQuant version 1.1 analysis following scanning of the gel with a Storm PhosphorImager. The abnormal eIF2α phosphorylation percentage with PP2Ac-B56α and PP2Ac-B56α-p at the 5-min time point is likely due to the undermeasurement of the initial eIF2α phosphorylation.