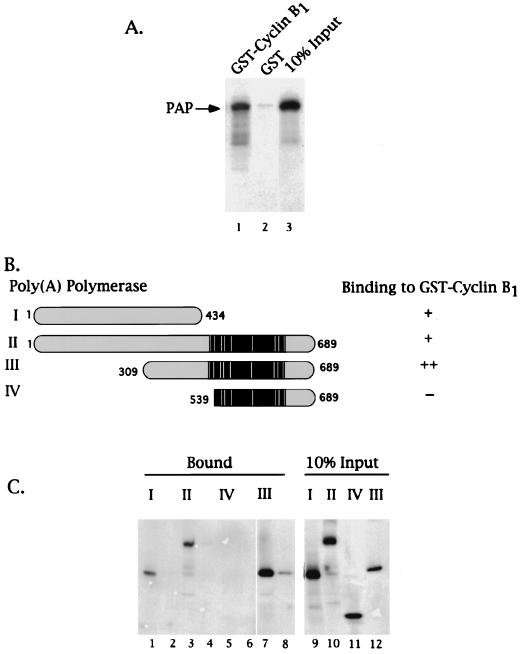
FIG. 2.
PAP binds cyclin B1 via residues N-terminal of its Ser-Thr-rich regulatory region. GST-cyclin B1 pull-down assays were performed using purified GST or GST fusion proteins bound to a glutathione matrix and in vitro-translated 35S-labeled PAPs (2 μl). (A) Autoradiogram of the eluates of either GST-cyclin B1 (lane 1) or GST (lane 2) glutathione matrices and 10% of the input PAP I (lane 3). An arrow on the left indicates the position of PAP I. (B) Schematic representation of PAP species used in the assay depicted in panel C and summary of results. The black region indicates the Ser-Thr-rich region, and the white bars indicate the sites for cdk phosphorylation. The bipartite nuclear localization signal sequences are boxed in gray. A plus sign indicates observed binding, two plus signs indicate strongest binding, and a minus sign indicates no binding was observed. (C) Autoradiogram of 35S-labeled PAPs bound to either GST-cyclin B1 (lanes 1, 3, 5, and 7) or GST (lanes 2, 4, 6, and 8). Lanes 9 to 12 are 10% of the input PAPs. The roman numerals indicate the PAP species used as graphically represented in panel C.