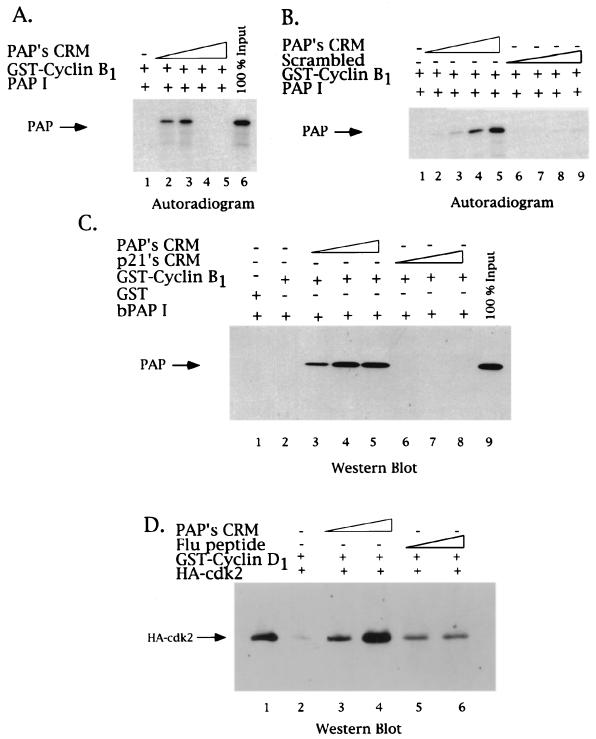
FIG. 6.
PAP's CRM both activates and represses PAP binding to cyclin B1. The effect of the 8-mer PAP CRM-derived peptide in GST-cyclin-PAP pull-down assays was tested. (A) GST-cyclin B1 glutathione matrices were incubated with in vitro-translated 35S-labeled PAP (1 μl) in the absence (lane 1) or presence of increasing amounts of PAP's CRM peptide (9, 18, 36, and 72 μM; lanes 2, 3, 4, and 5, respectively). Samples were washed, and bound proteins were analyzed by SDS–10% PAGE and autoradiography. An arrow on the left indicates the position of PAP. A total of 100% of the 35S-labeled PAP used in each reaction is found in lane 6. (B) Concentration dependence of the PAP CRM stimulatory effect. Lower concentrations of PAP's CRM peptide were used in binding assays similar to those in panel A (2.25, 4.5, 9, and 18 μM; lanes 2, 3, 4, and 5, respectively), as well as identical amounts of the 8-mer peptide of scrambled sequence (lanes 6, 7, 8, and 9). (C) CRM enhancement of PAP-cyclin B1 binding reflects a direct interaction. GST-cyclin B1 glutathione matrices were incubated with purified bacterial PAP in the presence of increasing amounts (2, 8.6, and 17 μM) of either PAP's CRM (lanes 3, 4, and 5), p21's CRM (lanes 6, 7, and 8), or no peptide (lane 1). Samples were subjected to SDS-PAGE and subsequent Western blot analysis using a PAP polyclonal antibody. (D) PAP's CRM can enhance cyclin-cdk association. GST-cyclin D1 glutathione matrices were incubated with purified HA-tagged cdk2 in the presence of increasing amounts (9 and 18 μM) of either PAP's CRM (lanes 3 and 4), a control peptide (lanes 5 and 6), or no peptide (lane 2). The presence of cdk2 in the eluates was detected by Western blot analysis using a monoclonal antibody against the HA epitope. Lane 1 contains the amount of HA-tagged cdk2 used.