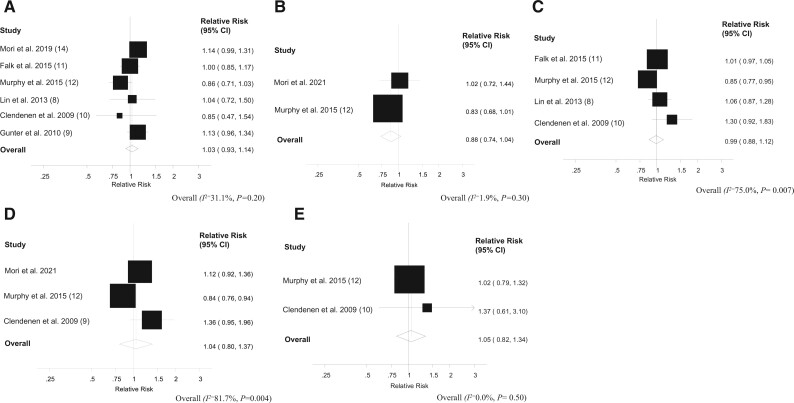
Figure 1.
Dose-response analysis between circulating estradiol, estrone, and colorectal cancer risk. A) Estradiol and colorectal cancer, per 5 pg/mL. B) Estradiol and colon cancer, per 5 pg/mL. C) Estrone and colorectal cancer, per 10 pg/mL. D) Estrone and colon cancer, per 10 pg/mL. E) Estrone and rectal cancer, per 10 pg/mL. The average of the natural logarithm of the relative risks was estimated, and the relative risk from each study was weighted using random effects weighting. A 2-tailed P < .05 was considered statistically significant. Heterogeneity between studies was quantitatively assessed by the Q test and I2. The black squares represent the odds ratios of the individual studies and the error bars their 95% confidence intervals (CIs). The area of the black squares reflects the weight each trial contributes in the meta-analysis.