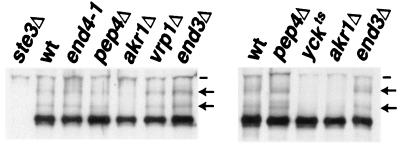
FIG. 1.
Effects of the endocytosis-defective mutants on Ste3p constitutive ubiquitination. Receptor ubiquitination was assessed in wild-type (wt) and mutant MATα GAL1-STE3 strains of two different genetic backgrounds. Strains isogenic to the wild-type strain NDY341 (left panel) include the temperature-sensitive end4-1 mutant (NDY342), as well as pep4Δ (NDY356), akr1Δ (NDY788), vrp1Δ (NDY1040), and end3Δ (NDY1046) mutants. In addition, the isogenic ste3Δ strain NDY343 was used as control for the specificity of Ste3p antibodies. Strains isogenic to NDY877 (wild-type MATα GAL1-STE3) (right panel) include the yck1Δ yck2ts double mutant (NDY913) and pep4Δ (NDY1080), akr1Δ (NDY1083), and end3Δ (NDY1118) strains. Protein extracts were prepared from cell cultures, following a 2-h Ste3p expression period, induced with galactose addition to log-phase cultures growing in raffinose medium. Fifteen minutes before cells were collected for extract preparation, cultures of cells isogenic to NDY341 (left panel) or to NDY877 (right panel) were shifted from 25°C to either 37 or 30°C, respectively. Protein extracts were treated with potato acid phosphatase (see Materials and Methods) to remove heterogeneity in gel migration which results from the heterogeneous phosphorylation of Ste3p and then subjected to SDS-PAGE and Western blotting with Ste3p-specific antibodies. Arrows indicate the positions of the mono- and diubiquitinated receptors; dashes indicate the positions of proteins that cross-react with the Ste3p antibodies.