Graphical Abstract
This editorial refers to ‘Compensatory post-diuretic renal sodium reabsorption is not a dominant mechanism of diuretic resistance in acute heart failure ’, by Z.L. Cox et al., https://doi.org/10.1093/eurheartj/ehab620.
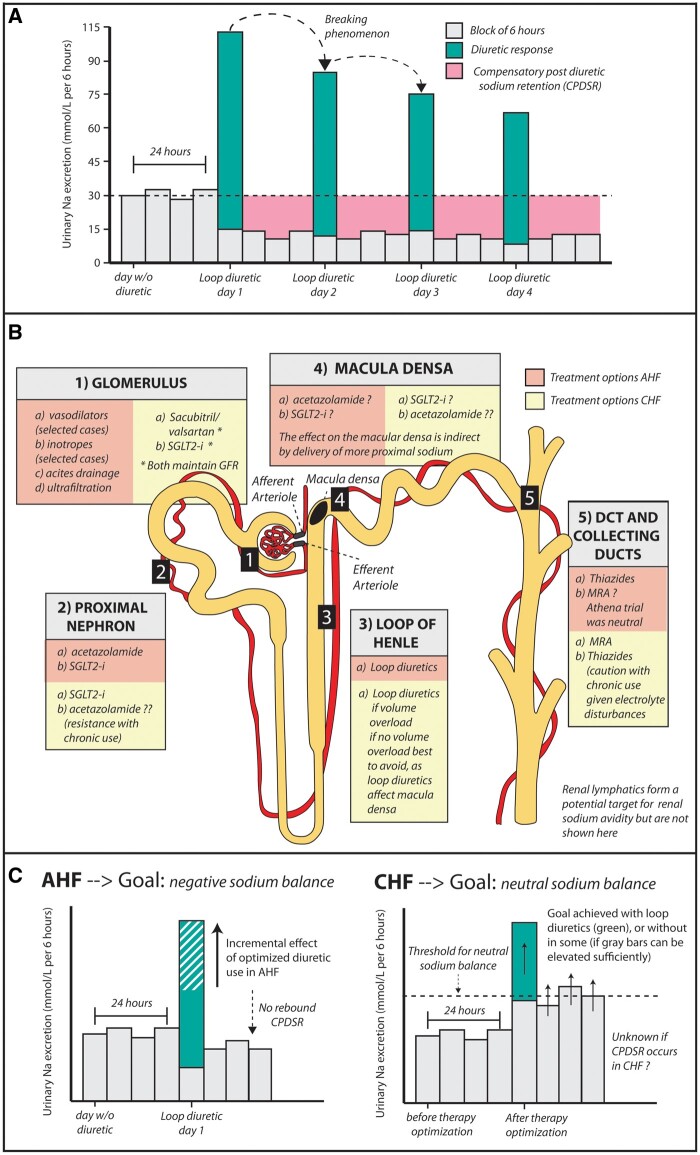
In those presenting with acute heart failure (AHF), congestion is the prevailing symptom in the majority of patients. While circulatory capacitance changes can provoke or worsen a clinical picture of congestion, the majority of patients are also volume overloaded,1 indicating excessive sodium and fluid retention in the intra- and extravascular space. Not surprisingly, removing this additional sodium and fluid is the main objective in these patients with AHF. The only way we remove this excessive sodium and fluid (when not using extracorporeal circulation) is by stimulating the kidneys with diuretics to release more sodium and fluid.1 Failure of the kidneys to inflict sufficient diuresis/natriuresis to achieve decongestion is perhaps one of the broader definitions of diuretic resistance, which is associated with poor clinical outcome.2 Numerous factors contribute to diuretic resistance, but compensatory sodium retention in the time frame after the loop diuretic effect has worn off is often thought to be and commonly taught to be a key factor (Graphical Abstract, panel A).3 Studies in euvolaemic healthy individuals have shown that administration of loop diuretics invokes compensatory post-diuretic sodium retention (CPDSR),4 a mechanism which is not mitigated by blocking the effect of angiotensin II by using captopril or by blocking an adrenergic effect by using prazosin.5,6 However, intravenous volume repletion in healthy individuals undergoing loop diuretic administration seems to mitigate CPDSR,7 indicating that extracellular volume (ECV) depletion seems to partially control this mechanism. As such, it is questionable whether CPDSR plays a role in evoking diuretic resistance in patients with AHF and clear volume overload.
Summary findings (A) Pharmacological effect of loop diuretics in healthy individuals showing CPDSR and the breaking phenomenon. (B) Potential therapies targeting sodium avidity in AHF and CHF. (C) Theoretical effect of therapies used in AHF and CHF on renal sodium excretion throughout the day.
In this issue of the European Heart Journal, Cox and colleague provide an interesting analysis of a prospective single-centre cohort study, designed to investigate the mechanism of diuretic resistance in patients with AHF.8 First, in healthy participants, the authors confirmed prior observations showing CPDSR in non-volume-overloaded healthy individuals.4 Secondly, and more importantly, the authors investigated CPDSR in patients admitted with AHF and signs of volume overload. The study consists of 462 individual diuretic administrations in 285 unique patients. They used a 6 h timed urine collection after loop diuretic administration to capture the diuretic-induced natriuresis, followed by a 18 h timed urine collection (once the loop diuretic effect has worn off) to capture CPDSR. Because these are real-world AHF patients and some clinicians prescribed multiple doses of loop diuretics during one day, the authors distinguished patients with only one loop diuretic administration per day (the cleanest cohort, defined as the measured cohort) vs. patients with multiple administrations (a less clean cohort, defined as the calculated cohort). Both in the measured and the calculated cohort, the authors show that loop diuretics induce a significant increase in natriuresis. However, in volume-overloaded AHF patients, the remaining 18 h of the day CPDSR did not seem to occur as natriuresis was similar in this time frame compared with that in healthy individuals. Because patients were placed on a 3 g salt-restrictive diet, the authors calculated the patients in which loop diuretics produced a negative sodium balance vs. a positive sodium balance (the latter being more ‘diuretic resistant’). Patients who attained a negative sodium balance not only had a larger 6 h diuretic-induced natriuresis but also a larger 18 h post-diuretic natriuresis, arguing against the existence of CPDSR in these patients, as there was a positive correlation between diuretic-induced and subsequent spontaneous natriuresis. In patients with a positive sodium balance, both the 6 h and the 18 h urine collection showed a diminished natriuresis. When similar patients to these latter patients were randomized to higher doses of loop diuretics or adding thiazides, the post-diuretic natriuresis rose three-fold, while the subsequent 18 h spontaneous natriuresis remained virtually unchanged, thereby showing that in patients with poor diuretic response, a therapy intensification to incremental diuretic doses or combinational diuretics can bolster diuretic efficacy without inflicting CPDSR. While Cox and colleagues provide a nice analysis, several limitations should be taken into account, such as the single-centre design, the double or triple inclusion of several patients (generating some bias), the absence of compelling data between the absence of CPDSR and the volume status of the patient, and the absence of sequential day determinations (does CPDSR occur on later days, when the breaking phenomenon occurs). Additionally, the result should not be extrapolated to patients with stable chronic heart failure (CHF; who have limited or no volume overload).
Another important prevailing message from this analysis is that the baseline intrinsic renal sodium avid state of the heart failure patients seems to be a major determinant of diuretic resistance. Indeed, the pre-diuretic urinary sodium concentration seems to determine both diuretic-induced natriuresis and spontaneous natriuresis (once the diuretic effect has worn off). Additionally, patients with a positive sodium balance seem to have a very pronounced sodium avid state. This observation in AHF is consistent with our recent report showing that in patients with stable CHF the morning (pre-diuretic) spot sodium concentrations were associated with the risk of being admitted for AHF, wth patients manifesting with a low urinary spot sodium concentration (more pronounced renal sodium avid state) having a higher risk of being admitted with AHF.9 In both cases, the intrinsic renal sodium avidity sets the basal rate of natriuresis, thus relatively insufficient natriuresis in the setting of volume overload can primarily be countered with increasing dosages and frequencies of diuretic therapy.
So what drives renal sodium avidity in heart failure and how can we leverage this knowledge into therapeutic advances if baseline renal sodium avidity is such an important target in heart failure? The kidneys are a specialized vascular organ receiving around a fifth of cardiac output. Understanding alterations that occur in this unique vascular structure (glomerular capillaries flanked by an afferent and efferent arteriole in serial structure with the more distal peri-tubular network) and the paralleling of renal tubules in the setting of heart failure help to determine causes and potential therapies targeting renal sodium avidity in heart failure (Graphical Abstract, panel B).10 First, increased filling pressures, a decrease in cardiac output, and elevation in abdominal pressures result in diminished glomerular renal blood flow (RBF) and elevated glomerular hydrostatic capillary pressures at the level of the glomerulus.11 Hydrostatic capillary pressures are elevated both due to passive transmitted pressure in the renal veins (elevated filling pressures) and through neurohormonal activation (more vasoconstriction of the efferent vs. afferent arteriole).12 Chronic elevated glomerular hydrostatic pressures can result in accelerated nephron loss occurring in heart failure.2 A decrease in RBF results in a net increased filtration (increased filtration fraction) across the glomerular capillaries in the Bowman’s space.13 Secondly, because a state of increased filtration fraction (heart failure) results in enhanced oncotic pressure in the peritubular network (often in conjunction with lower hydrostatic pressures due the efferent arteriole vasoconstriction), this stimulates proximal nephron sodium retention (tubulo-glomerular balance).13 Normally, the proximal nephron reabsorbs ∼65% of all filtered sodium in the renal tubules, but this can be elevated in heart failure due to enhanced proximal nephron sodium avidity secondary to the increased filtration fraction. Neurohormonal activation (and certain comorbidities such as obesity and diabetes) can also result in up-regulation of proximal sodium transporters (Na/H-exchanger of Na-glucose linked transporter), further worsening proximal sodium retention.10 Thirdly, diminished tubular flow in the thick ascending loop of Henle (due to more proximal sodium retention and low RBF), combined with neurohormonal activation, results in renal sodium avidity in the loop of Henle.10 Sodium retention in this segment also further builds the medullar interstitial gradient which drives free water reabsorption. Fourthly, enhanced renal sodium avidity more proximal to the macula densa results in less chloride uptake by the macula densa, thereby evoking signals of ECV depletion such as neurohormonal activation or tubuloglomerular feedback (vasodilation of the afferent arteriole, leading to enhanced hydrostatic glomerular capillary pressures and potentially accelerated nephron loss). Fifthly, reduced tubular flow in the distal nephron (distal convoluted tubule and collecting ducts), elevated aldosterone concentrations, and the breaking phenomenon (hypertrophy of distal tubular cells) enhance distal nephron sodium avidity.10 In AHF the goal of therapy is to induce a net negative sodium and fluid balance eventually leading to decongestion, while in CHF the goal is to maintain a neutral balance, preventing occurrence of decompensation (Graphical Abstract, panel C). Therapies in AHF and CHF that target the five aforementioned renal sites determining enhanced sodium avidity are reflected in the Graphical Abstract, panel B. In CHF, due to medical therapy optimization, not all patients require a loop diuretic (if medical therapies are capable of inducing a state of sufficient baseline renal natriuresis). However, in patients with CHF requiring a loop diuretic, we have recently shown that termination of this loop diuretic results in a significant drop in natriuresis.14
Cox and colleagues provide interesting data in the setting of AHF showing that CPDSR does not influence diuretic resistance. Furthermore, they provide the insight that in AHF patients with a poor diuretic response and positive sodium balance, the use of targeted diuretics can bolster diuretic response without inflicting CPDSR. Additional studies will need to determine which diuretic strategy (an increase in loop diuretic dose, adding acetazolamide, adding an SGLT2-I, adding thiazide, etc.) is most appropriate in which patients. The ongoing ADVOR (Acetazolamide in patients with Decompensated heart failure and Volume OveRload) trial, for instance, will test the effect of acetazolamide for this purpose in AHF.15
Funding
P.M. is supported by 1 year support from the Belgian American Educational Foundation (BAEF) and 1 year support from the Frans Van de Werf Fund. W.H.W.T is partially supported by grants from the National Institutes of Health (R01HL126827 and R01HL146754).
Conflict of interest: W.H.W.T. is a consultant for Sequana Medical A.G., Owkin Inc., Relypsa Inc., PreCARDIA Inc., Cardiol Therapeutics Inc., and Genomics plc, and has received honoraria from Springer Nature for authorship/editorship and the American Board of Internal Medicine for exam writing and committee participation—all unrelated to the subject and contents of this paper. All other authors have no relationships to disclose in relation to this manuscript.
Contributor Information
Pieter Martens, Department of Cardiology, Ziekenhuis Oost-Limburg, Genk, Belgium; Department of Cardiovascular Medicine, Cleveland Clinic, Cleveland, OH, USA.
W H Wilson Tang, Department of Cardiovascular Medicine, Cleveland Clinic, Cleveland, OH, USA.
Wilfried Mullens, Department of Cardiology, Ziekenhuis Oost-Limburg, Genk, Belgium; Biomedical Research Institute, Faculty of Medicine and Life Sciences, Hasselt University, Diepenbeek, Belgium.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-la Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:137–155. [DOI] [PubMed] [Google Scholar]
- 2. Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, Tang WHW, Skouri H, Verbrugge FH, Orso F, Hill L, Ural D, Lainscak M, Rossignol P, Metra M, Mebazaa A, Seferovic P, Ruschitzka F, Coats A. Evaluation of kidney function throughout the heart failure trajectory: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:584–603. [DOI] [PubMed] [Google Scholar]
- 3. Ellison DH, Felker GM. Diuretic treatment in heart failure. N Engl J Med 2017;377:1964–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilcox CS, Mitch WE, Kelly RA, Skorecki K, Meyer TW, Friedman PA, Souney PF. Response of the kidney to furosemide. I. Effects of salt intake and renal compensation. J Lab Clin Med 1983;102:450–458. [PubMed] [Google Scholar]
- 5. Kelly RA, Wilcox CS, Mitch WE, Meyer TW, Souney PF, Rayment CM, Friedman PA, Swartz SL. Response of the kidney to furosemide. II. Effect of captopril on sodium balance. Kidney Int 1983;24:233–239. [DOI] [PubMed] [Google Scholar]
- 6. Wilcox CS, Guzman NJ, Mitch WE, Kelly RA, Maroni BJ, Souney PF, Rayment CM, Braun L, Colucci R, Loon NR. Na+, K+, and BP homeostasis in man during furosemide: effects of prazosin and captopril. Kidney Int 1987;31:135–141. [DOI] [PubMed] [Google Scholar]
- 7. Hammarlund MM, Odlind B, Paalzow LK. Acute tolerance to furosemide diuresis in humans. Pharmacokinetic–pharmacodynamic modeling. J Pharmacol Exp Ther 1985;233:447–453. [PubMed] [Google Scholar]
- 8.Cox ZL, Rao VS, Ivey-Miranda JB, Moreno-Villagomez J, Mahoney D, Ponikowski P, Biegus J, Turner JM, Maulion C, Bellumkonda L, Asher JL, Parise H, Wilson PF, Ellison DH, Wilcox CS, Testani JM. Compensatory post-diuretic renal sodium reabsorption is not a dominant mechanism of diuretic resistance in acute heart failure. Eur Heart J 2021;doi:10.1093/eurheartj/ehab620. [DOI] [PMC free article] [PubMed]
- 9. Martens P, Dupont M, Verbrugge FH, Damman K, Degryse N, Nijst P, Reynders C, Penders J, Tang WHW, Testani J, Mullens W. Urinary sodium profiling in chronic heart failure to detect development of acute decompensated heart failure. JACC Heart Fail 2019;7:404–414. [DOI] [PubMed] [Google Scholar]
- 10. Mullens W, Verbrugge FH, Nijst P, Tang WHW. Renal sodium avidity in heart failure: from pathophysiology to treatment strategies. Eur Heart J 2017;38:1872–1882. [DOI] [PubMed] [Google Scholar]
- 11. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009;53:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verbrugge FH, Dupont M, Steels P, Grieten L, Swennen Q, Tang WH, Mullens W. The kidney in congestive heart failure: ‘are natriuresis, sodium, and diuretics really the good, the bad and the ugly?’. Eur J Heart Fail 2014;16:133–142. [DOI] [PubMed] [Google Scholar]
- 13. Arrizurieta-Muchnik EE, Lassiter WE, Lipham EM, Gottschalk CW. Micropuncture study of glomerulotubular balance in the rat kidney. Nephron 1969;6:418–436. [DOI] [PubMed] [Google Scholar]
- 14. Dauw J, Martens P, Tersalvi G, Schouteden J, Deferm S, Gruwez H, De MB, Nijst P, Dupont M, Mullens W. Diuretic response and effects of diuretic omission in ambulatory heart failure patients on chronic low-dose loop diuretic therapy. Eur J Heart Fail 2021;23:1110–1119. [DOI] [PubMed] [Google Scholar]
- 15. Mullens W, Verbrugge FH, Nijst P, Martens P, Tartaglia K, Theunissen E, Bruckers L, Droogne W, Troisfontaines P, Damman K, Lassus J, Mebazaa A, Filippatos G, Ruschitzka F, Dupont M. Rationale and design of the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial. Eur J Heart Fail 2018;20:1591–1600. [DOI] [PubMed] [Google Scholar]