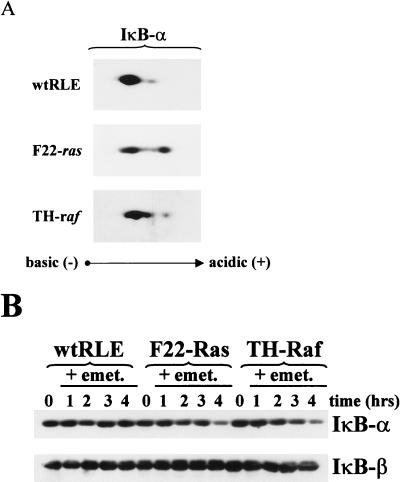
FIG. 3.
IκB-α protein is more phosphorylated and has a shorter half-life in F22-Ras and TH-Raf cells than in wt RLEs. (A) Phosphorylation state. Cytoplasmic extracts (40 μg) from exponentially growing wt, F22-Ras and TH-Raf RLEs, treated for 1 h with 40 μM calpain inhibitor I to inhibit IκB-α degradation, were resolved on isoelectric focusing gels, separated according to molecular weight by SDS-PAGE, and subjected to immunoblot analysis using an antibody preparation raised against the IκB-α product (SC-371). (B) Half-life of decay. Exponentially growing RLEs were treated with the protein synthesis inhibitor emetine (emet; 10 μg/ml) for 1 to 4 h. Cytoplasmic extracts (20 μg) were then subjected to immunoblot analysis for IκB-α as described above.