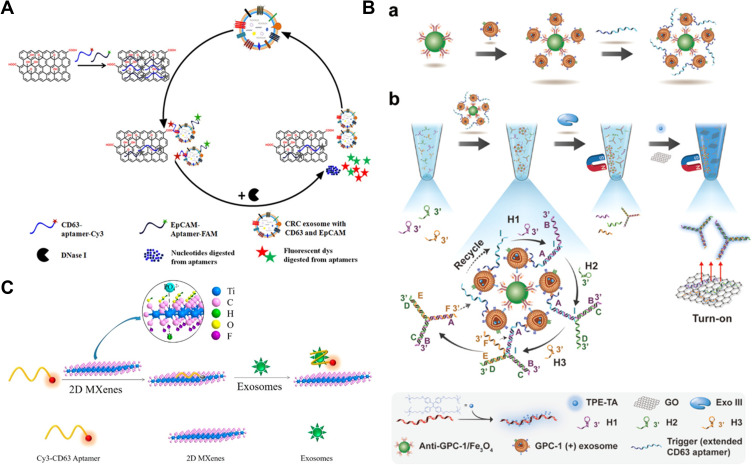
Figure 4.
(A) Schematic of enzyme-aided fluorescence amplification based on GO-DNA aptamer interactions for exosome detection. Reprinted with permission from Wang H, Chen H, Huang Z, Li T, Deng A, Kong J. DNase I enzyme-aided fluorescence signal amplification based on graphene oxide-DNA aptamer interactions for colorectal cancer exosome detection. Talanta. 2018;184:219–226. Copyright 2018 Elsevier B.V.102 (B) Schematic of homogeneous magneto-fluorescent nanosensor for tumor-derived exosome isolation and analysis. (a) Tumor-derived exosomes are specifically captured by GPC-1 antibody coated magnetic beads and subsequently bind with extended CD63 aptamers, forming a bead−exosome−aptamer complexes. (b) Captured exosomes are detected in a homogeneous solution by aptamer-triggered DNA TWJs cyclic assembly strategy along with TPE-TA and the GO-based “turn-on” fluorescent system. Reprinted with permission from Li B, Pan W, Liu C, et al. Homogenous magneto-fluorescent nanosensor for tumor-derived exosome isolation and analysis. ACS Sens. 2020;5:2052–2060. Copyright 2020 American Chemical Society.103 (C) Schematic of Cy3-CD63 aptamer was mixed with MXenes aqueous solution and then added exosomes. Reprinted with permission from Zhang Q, Wang F, Zhang H, Zhang Y, Liu M, Liu Y. Universal Ti3C2 MXenes based self-standard ratiometric fluorescence resonance energy transfer platform for highly sensitive detection of exosomes. Anal Chem. 2018;90:12737–12744. Copyright 2018 American Chemical Society.105