Figure 5.
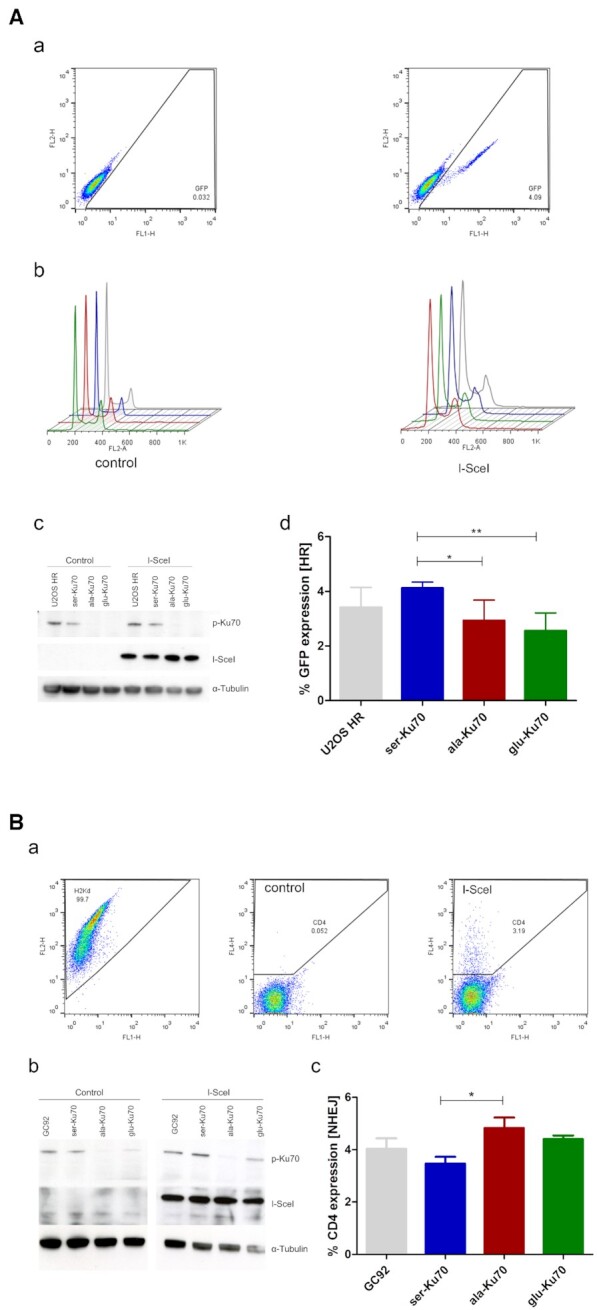
Ser-Ku70 stimulates HR activity while ala-Ku70 promotes DNA DSB distal end junctions. (A) HR activity and the cell cycle were assessed by flow cytometry of U2OS cell line containing the integrated reporter construct pDR-GFP (a and b). This cell line was transfected using ser-, ala-, or glu-Ku70 vectors as described in the methods section. (c) pKu70 expression was verified by western blot and is shown as cropped blots. The reporter is composed of two inactive eGFP genes. The upstream GFP gene is truncated; the gene downstream of the promoter has an integrated I-SceI cleavage site and is therefore inactive. Upon I-SceI expression, as verified by western blotting (c) (α-tubulin was used as the loading control), the cleaved GFP gene recombines with the truncated GFP gene on the sister chromatid, resulting in the expression of GFP, which was measured by flow cytometry 72h after I-SceI transfection (exemplified in a). The results (d) represent values that were calculated as follows: (I-SceI – transfection events) – (control – transfection events). n = 6. Unpaired t-test, *P < 0.01. (B) NHEJ activity was measured (a) using the human fibroblast cell line GC92 (SV40-transformed) expressing ser-, ala-, or glu-Ku70 [controlled by western cropped (b) blots] that contains the intrachromosomally integrated pCOH-CD4 (cohesive ends) reporter construct. Upon cleavage by I-SceI, a fragment containing the H2Kd and CD8 genes was excised. Re-joining of the flanking ends by NHEJ brought the pCMV promoter closer to the CD4 gene, which promoted its expression. (a) CD4 expression on the cell surface was quantified by flow cytometry 72 h after I-SceI transfection. (c) The results represent values that were calculated as follows: (I-SceI – transfection events) – (control – transfection events). n = 4. Unpaired t-test, *P < 0.01. Differences between values of cells expressing ser-Ku70 or glu-Ku70 are statisticaly non significant.
