Abstract
Background
Various epidemiological and experimental studies propose that helminths could play a preventive role against the progression of type 2 diabetes mellitus (T2DM). T2DM induces microvascular and large vessel complications mediated by elevated levels of angiogenic factors and soluble receptor for advanced glycation end product (RAGE) ligands. However, the interactions between helminths and host angiogenic factors and RAGE ligands are unexplored.
Methods
To assess the relationship between a soil-transmitted helminth, Strongyloides stercoralis (Ss), and T2DM, we measured plasma levels of vascular endothelial growth factor (VEGF)–A, -C, and -D; angiopoietins 1 and 2 (Ang-1 and Ang-2); and their receptors VEGF-R1, -R2, and -R3 as well as soluble RAGE (sRAGE) and their ligands advanced glycation end products (AGEs), S100A12, and high mobility group box 1 (HMGB-1) in individuals with T2DM with or those without Ss infection. In Ss-infected individuals, we also measured the levels of aforementioned factors 6 months following anthelmintic therapy.
Results
Ss-infected individuals exhibited significantly decreased levels of VEGF-A, VEGF-C, VEGF-D, Ang-1, and Ang-2 and their soluble receptors VEGF-R1, -R2, and -R3, that increased following anthelmintic therapy. Likewise, Ss-infected individuals exhibited significantly decreased levels of AGEs and their ligands sRAGE, S100A12, and HMGB-1, which reversed following anthelmintic therapy.
Conclusions
Our data suggest that Ss infection could play a beneficial role by limiting or delaying T2DM-related vascular complications.
Keywords: Strongyloides stercoralis infection, type 2 diabetes mellitus, angiogenic factors, RAGE ligands, VEGFs and receptors, S100A12, HMGB-1
Helminth infections are associated with decreased levels of angiogenic factors and their receptors, advanced glycation end products, and their ligands. These alterations are reversed following anthelmintic treatment. Strongyloides stercoralis infection could play a beneficial role by limiting or delaying the type 2 diabetes mellitus–related vascular complications.
Human and animal studies have demonstrated that helminth infections are associated with a decreased prevalence of metabolic syndrome or type 2 diabetes mellitus (T2DM) [1]. With concurrent diminution of parasitism in industrialized countries, the incidence of metabolic syndrome, allergy, and autoimmune diseases are increasing enormously [2]. T2DM is a chronic condition and considered as a major cause of mortality and morbidity because of microvascular (such as retinopathy, nephropathy, and neuropathy) and large vessel (coronary heart disease, peripheral vascular disease, and stroke) complications [3].
Vascular endothelial growth factor (VEGF) and the angiopoietins (Ang)/Tie-2 are 2 families of vascular-associated molecules that are crucial for vessel formation and maturation [4–6]. VEGFs exert their biological outcomes through their interaction with specific (VEGF-R) receptors [7]. Abnormal angiogenesis in diabetes is likely mediated in part by exuberant VEGF-A family-induced signaling, which induces diabetic retinopathy and nephropathy in diabetic patients [8–10]. Ang-1 supports endothelial cell survival, alleviates endothelial interactions with supporting cells, and controls vascular permeability [11]. In contrast, Ang-2 has been suggested as a natural antagonist of Ang-1 that destabilizes the vasculature [12], while concurrently enabling endothelial cell migration and proliferation with VEGF [11, 12]. Previous studies revealed that plasma/serum samples exhibited increased VEGF concentrations in patients with diabetes compared with nondiabetic patients, and hyperglycemia alters the levels of circulating angiogenic growth factors, findings that suggest their key role in pathogenesis in T2DM [11, 13]. Similar to VEGF, increased plasma levels of angiopoietins have been reported in patients with diabetes, and the increased levels were associated with endothelial damage/dysfunction [11, 14].
T2DM has been mainly associated with vascular calcification through a variety of mechanisms, among which oxidative stress, hyperglycemia, and hypercalcemia play a key role [15]. Studies have also determined that advanced glycation end products (AGEs) and the receptors for advanced glycation end products (RAGEs) play a role in vascular calcification [16]. AGEs are biochemical end products of nonenzymatic glycosylation in diabetic patients with poor glycemic control. AGEs lead to extracellular matrix (ECM) enlargement in diabetic tissue fibrosis and resultant biochemical modification in protein structure and function [8]. The AGE/RAGE signaling cascade has been determined as a controlling loop whereby outcomes due to an augmented fibrosis, RAGE expression, and oxidative stressors are elicited [17, 18]. Other RAGE ligands, such as SA100A12 and high mobility group box 1 (HMGB-1), are involved in the inflammatory milieu of T2DM and its resultant complications [19, 20].
We hypothesized that helminth infections could alter the levels of angiogenic factors and AGE and its ligands that could potentially lead to the amelioration of diabetic vascular complications. To this end, we measured the circulating levels of VEGF-A, VEGF-C, VEGF-D, Ang-1, and Ang-2; and AGE and its ligands, soluble RAGE (sRAGE) S100A12 and HMGB-1, in individuals with T2DM with or without concurrent Strongyloides stercoralis (Ss) infection. We also examined the effect of anthelmintic therapy after 6 months on the aforesaid parameters in Ss-infected individuals.
MATERIALS AND METHODS
Ethics Statement
All participants were examined as part of a natural history study protocol (12-I-073) approved by institutional review boards of the National Institute of Allergy and Infectious Diseases (United States) and the National Institute for Research in Tuberculosis (India), and informed written consent was obtained from all participants.
Study Population
We recruited 118 individuals consisting of 60 clinically asymptomatic Ss-infected individuals with T2DM (hereafter Ss+), and 58 individuals with T2DM and no Ss infection (hereafter Ss–) in Kanchipuram District, Tamil Nadu, South India. All study participants had no previous history of helminth infections or anthelmintic treatment and were human immunodeficiency virus seronegative. This is the same cohort of individuals previously described in our reports [21, 22].
Measurement of Anthropometric and Biochemical Parameters
Anthropometric measurements, including height, weight, and waist circumference, and biochemical parameters, including plasma random blood glucose, glycated hemoglobin (HbA1c), urea, creatinine, alanine aminotransferase, and aspartate aminotransferase were obtained using standardized techniques as detailed elsewhere [23].
Parasitological Examination and Anthelmintic Treatment
Strongyloides stercoralis infection was diagnosed by the presence of immunoglobulin G antibodies to the recombinant NIE antigen as described previously [24, 25]. Stool microscopy was used to exclude the presence of other intestinal helminth infections. Filarial infection was excluded in all study participants by virtue of being negative in tests for circulating filarial antigen. All Ss+ individuals were treated with a single dose of ivermectin (12 mg) and albendazole (400 mg) and follow-up blood draws were obtained 6 months later. All of our treated subjects were serology negative at 6 months after treatment.
Determination of T2DM Status
Based on American Diabetes Association criteria, T2DM was confirmed by an HbA1c value of 6.5% or greater and a random blood glucose of >200 mg/dL. Overnight fasting samples were used to measure all biochemical parameters with exception of random blood glucose. All diabetic individuals were newly diagnosed, not on any antidiabetic medication at the time of blood draw, and without any known complications or comorbidities. All individuals were referred to the primary healthcare center for diabetic treatment. The individuals who had previous history of diabetes complications, neurological problems, renal problems, and cardiovascular-related problems were excluded from the study.
Measurement of Angiogenic Factors and RAGE Ligands
Systemic levels of VEGF-A, VEGF-C, VEGF-R1, VEGF-R2, and VEGF-R3 were determined by means of the Duoset enzyme-linked immunosorbent assay (ELISA) development system (R&D Systems). Quantikine ELISA kit (R&D Systems) was used for measuring VEGF-D. The lowest detection limits were as follows: VEGF-A, 31.25 pg/mL; VEGF-C, 62.5 pg/mL; VEGF-D, 62.5 pg/mL; VEGF-R1, 125 pg/mL; VEGF-R2, 31.25 pg/mL; VEGF-R3, 156.25 pg/mL. Levels of Ang-1 and Ang-2 were determined by means of the Duoset ELISA development system (R&D Systems). The assay range is 156.0–10 000 pg/mL for Ang-1 and 93.8–6000 pg/mL for Ang-2. Systemic levels of AGE (carboxymethyl lysine) were determined by means of the Cell Biolabs kit. sRAGE was determined by means of the Quantikine ELISA kit (R&D Systems), S100A12 levels were determined by means of the MBL kit and HMGB-1 using the Mybiosource kit. The lowest detection limits were as follows: AGE, 0.39 μg/mL; sRAGE, 78.12 pg/mL; S100A12, 20 pg/mL; and HMGB-1, 19.5 pg/mL.
Statistical Analysis
Geometric means (GMs) were used for measurements of central tendency. The Ss+ group was compared to the Ss– group by Mann–Whitney U tests and before and after treatment parameters were compared by Wilcoxon signed-rank test. Multiple comparisons were corrected using the Holm correction. Analyses were performed using GraphPad Prism version 8.4.3. (GraphPad, San Diego, California). Stata version 15 software (StataCorp, College Station, Texas) was used to perform the multiple logistic regression analysis.
RESULTS
Study Population Characteristics
The baseline demographic characteristics and biochemical parameters have been described previously [21, 22] and there were no significant differences in age, sex, body mass index (BMI), or other biochemical parameters between the 2 groups. The baseline and posttreatment demographics and biochemical parameters are shown in Supplementary Table 1.
Diminished Circulating Levels of Angiogenic Factors in Ss+ Individuals
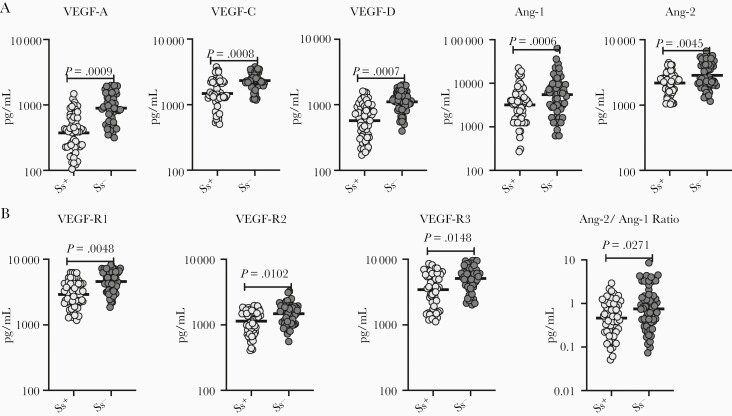
To evaluate the impact of Ss infection on angiogenic factors in T2DM, we measured the circulating levels of VEGF-A, VEGF-C, VEGF-D, Ang-1, Ang-2, VEGF-R1, VEGF-R2, and VEGF-R3 in Ss+ and Ss– individuals. As shown in Figure 1 and Supplementary Figure 2A and 2B (box plot), the levels (GMs) of VEGF-A (347.7 pg/mL in Ss+ vs 894.1 pg/mL in Ss–; P = .0009), VEGF-C (1482 pg/mL in Ss+ vs 2340 pg/mL in Ss–; P = .0008), VEGF-D (568.9 pg/mL in Ss+ vs 1106 pg/mL in Ss–; P = .0007), Ang-1 (3183 pg/mL in Ss+ vs 5509 pg/mL in Ss–; P = .0006), Ang-2 (2185 pg/mL in Ss+ vs 2856 pg/mL in Ss–; P = .0045), VEGF-R1 (2906 pg/mL in Ss+ vs 4581 pg/mL in Ss–; P = .0048), VEGF-R2 (1143 pg/mL in Ss+ vs 1481 pg/mL in Ss–; P = .0008), and VEGF-R3 (3444 pg/mL in Ss+ vs 5091 pg/mL in Ss–; P = .0008) were significantly lower in Ss+ compared to Ss– individuals. Next, we determined the ratio between the 2 antagonist angiopoietins. The ratio between Ang-2 and Ang-1 decreased in Ss+ compared with the Ss– individuals. As shown in Supplementary Figure 1A, the levels of the angiogenic factors for each subject are shown as spaghetti plots. In addition, we have shown the data for the comparison between Ss– and posttreatment individuals (Supplementary Figure 2A and 2B). Thus, Ss infection is associated with lower circulating levels of the angiogenic factors in Ss+ subjects with T2DM.
Figure 1.
Diminished circulating levels of angiogenic factors in Strongyloides stercoralis–infected (Ss+) individuals. A, Plasma levels of vascular endothelial growth factor (VEGF)–A, VEGF-C, VEGF-D, angiopoietin (Ang) I, and Ang-2 type 2 diabetes mellitus (T2DM) in Ss+ (n = 60) and S. stercoralis–uninfected (Ss–) (n = 58) individuals. B, Plasma levels of VEGF-R1, VEGF-R2, VEGF-R3, and Ang-2/Ang-1 ratio in T2DM in Ss+ (n = 60) and Ss– (n = 58) individuals. Each dot is an individual subject with the bar representing the geometric mean. Mann–Whitney U test with Holm correction for multiple comparisons was done. P values are multiplied by the number of parameters.
Diminished Circulating Levels of AGE and Its Ligands in Ss+ Individuals
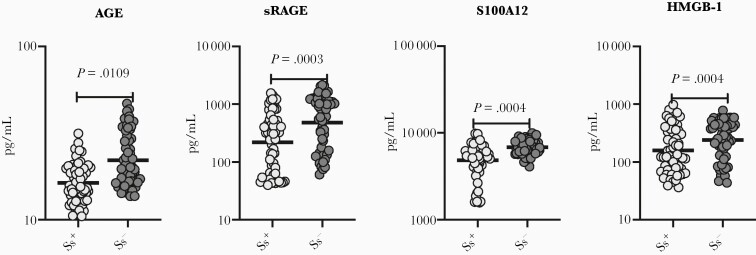
To evaluate the impact of Ss infection on AGE and its ligands in T2DM, we measured the circulating levels of AGE and their ligands (sRAGE, S100A12, and HMGB-1 in Ss+ and Ss– individuals. As shown in Figure 2 and Supplementary Figure 3C (box plot), Ss+ individuals had significantly lower levels (GMs) of AGE (16.35 vs 22.06 pg/mL; P = .0109), sRAGE (217.5 vs 480.7 pg/mL; P = .0003), S100A12 (4842 vs 6869 pg/mL; P = .0004), HMGB-1 (157.8 vs 240 pg/mL; P = .0004) when compared with Ss– individuals. As shown in Supplementary Figure 1B, the levels of the RAGE ligands for each subject are shown as spaghetti plots. In addition, we have shown the data for the comparison between Ss– and posttreatment individuals (Supplementary Figure 2C). Thus, Ss infection with T2DM is associated with lower circulating levels of AGE ligands.
Figure 2.
Diminished circulating levels of receptor for advanced glycation end product (RAGE) ligands in Strongyloides stercoralis–infected (Ss+) individuals. A, Plasma levels of advanced glycation end products (AGEs), soluble RAGE (sRAGE), S100A12, and high mobility group box 1 (HMGB-1) in Ss+ (n = 60) and S. stercoralis–uninfected (Ss–) (n = 58) individuals with type 2 diabetes mellitus. Each dot is an individual subject with the bar representing the geometric mean. Mann–Whitney U test with Holm correction for multiple comparisons was done. P values are multiplied by the number of parameters.
Changes in Circulating Levels of Angiogenic Factors Following Anthelmintic Treatment
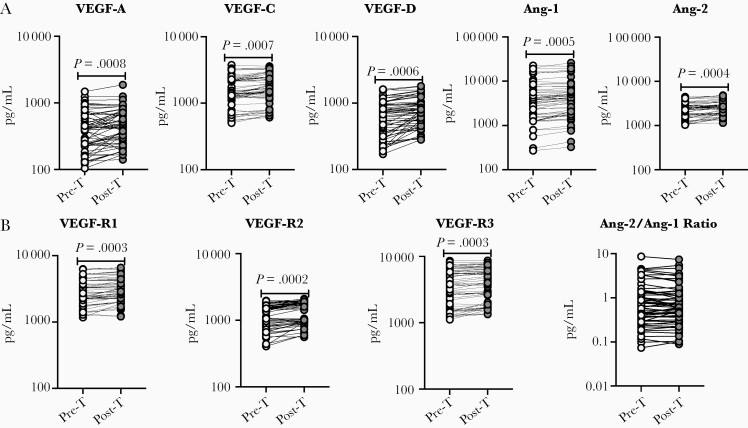
Next, we wanted to determine the effect of anthelmintic treatment on the levels of VEGF-A, VEGF-C, VEGF-D, Ang-1, Ang-2, VEGF-R1, VEGF-R2, and VEGF-R3, we measured angiogenic factors in Ss+ individuals following anthelmintic treatment. At 6 months following anthelmintic treatment, as shown in Figure 3, the posttreatment levels of VEGF-A (percentage increase of 15.6%; P = .0008), VEGF-C (percentage increase of 10.4%; P = .0007), VEGF-D (percentage increase of 22.5%; P = .0006), Ang-1 (percentage increase of 14.5%; P = .0005), Ang-2 (percentage increase of 11.5%; P = .0004), VEGF-R1 (percentage increase of 8%; P = .0003), VEGF-R2 (percentage increase of 14.2%; P = .0002), and VEGF-R3 (percentage increase of 7.9%; P = .0003) were significantly elevated when compared to pretreatment levels. The ratio levels of Ang-2/Ang-1 do not show any significant difference. Thus, anthelmintic treatment is associated with elevated levels of angiogenic factors.
Figure 3.
Elevated circulating levels of angiogenic factors following anthelmintic treatment. A, Plasma levels of vascular endothelial growth factor (VEGF)–A, VEGF-C, VEGF-D, angiopoietin (Ang) 1, and Ang-2 in individuals with type 2 diabetes mellitus (T2DM) infected with Strongyloides stercoralis (Ss+) pretreatment (Pre-T, n = 60) and 6 months following treatment (Post-T, n = 60) were measured. B, Plasma levels of VEGF-R1, -R2, -R3, and Ang-2/Ang-1 ratio in individuals with T2DM and Ss+ Pre-T (n = 60) and Post-T (n = 60) were measured. P values were calculated using the Wilcoxon matched pairs test.
Changes in Circulating Levels of AGE Ligands Following Anthelmintic Treatment
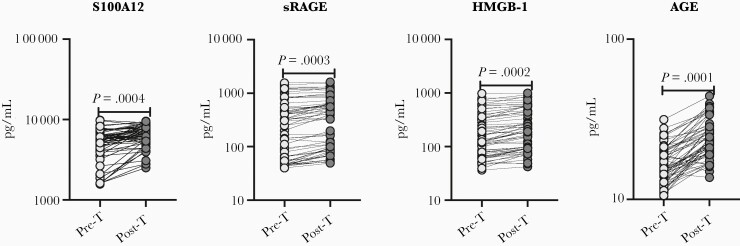
To determine the effect of anthelmintic treatment on circulating levels of AGE, sRAGE, S100A12, and HMGB-1, we measured the RAGE ligands in Ss+ individuals following anthelmintic treatment. At 6 months following anthelmintic treatment, as shown in Figure 4, the levels of AGE (percentage increase of 37.3%; P = .0001), sRAGE (percentage increase of 24%; P = .0003), S100A12 (percentage increase of 25.3%; P = .0004), and HMGB-1 (percentage increase of 23.1%; P = .0002) all increased following treatment. Thus, anthelmintic treatment is associated with elevated levels of AGE ligands.
Figure 4.
Elevated levels of receptor for advanced glycation end product (RAGE) ligands following anthelmintic treatment. Plasma levels of advanced glycation end products (AGEs), soluble RAGE (sRAGE), S100A12, and high mobility group box 1 (HMGB-1) in individuals with type 2 diabetes mellitus and Strongyloides stercoralis infection (Ss+) pretreatment (Pre-T, n = 60) and 6 months following treatment (Post-T, n = 60) were measured. P values were calculated using the Wilcoxon matched pairs test.
Multivariate Regression Analysis of Angiogenic Factor and AGE and Its Ligands
Multivariate regression analysis was performed to evaluate the impact of Ss infection on the diverse parameters measured in this study in individuals with T2DM. As indicated in Supplementary Table 1, after adjusting for the influence of age, sex, and BMI, VEGF-A, -C, and -D; Ang-1 and Ang-2; VEGF-R1, -R2, and -R3; and S100A12, sRAGE, HMGB-1, and AGE were all significantly impacted by Ss infection. Thus, our data confirm that Ss infection has a profound influence on various important parameters in individuals with T2DM, including levels of the angiogenic growth factors and RAGE and its ligands.
Principle Components Analysis Reveals Trends in Angiogenic Growth Factors and RAGE and Its Ligands
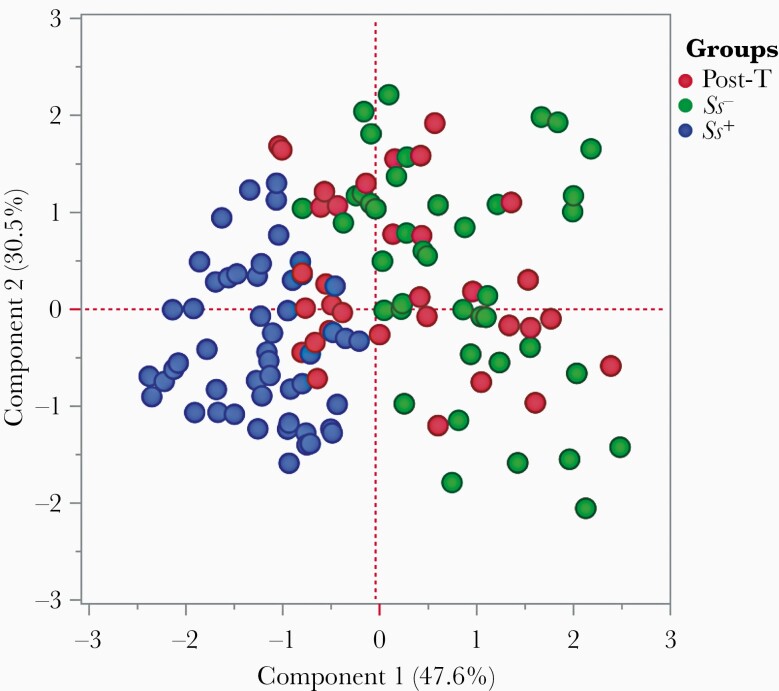
Principal components analysis (PCA) was used to visualize differences between the groups created on the entire data set. To visualize the clustering pattern of angiogenic growth factors and RAGE and its ligands in T2DM individuals with or without Ss infection. we performed PCA with angiogenic factors (VEGF-A, -C, and -D; VEGF-R1, -R2, and -R3; and Ang-1 and -2) and AGE and its ligands (sRAGE, S100A12, HMGB-1, and AGE). After excluding those factors with commonalities as low as 0.5, we assessed PCA-1 (VEGF-A, -C, -D, and -R1) and PCA-2 (Ang-1, HMGB-1, and sRAGE). As illustrated in Figure 5, PCA analysis showed that angiogenic growth factors and AGE and its ligands clusters varied between Ss+ and Ss– with T2DM and posttreatment (Ss+ with T2DM) individuals. The score plot of the first 2 components revealed 47.6% and 30.5% of overall variance, respectively.
Figure 5.
Principal components analysis (PCA) reveals trends in angiogenic growth factors and receptor for advanced glycation end products (RAGEs) and its ligands. PCA was performed to show the distribution of data from the combination of 3 groups: Strongyloides stercoralis–infected individuals (Ss+, blue circles); S. stercoralis–uninfected individuals (Ss–, green circles); and posttreatment (maroon circles). The PCA represents the 2 principal components of variation. PCA analysis was performed with angiogenic growth factors and RAGE and its ligands between Ss+ and Ss– individuals with type 2 diabetes mellitus and helminth infection posttreatment.
DISCUSSION
T2DM is a state of chronic inflammation that affects the function and structure of small and large blood vessels [26]. Helminth infections are well known modulators of immune responses due to their tendency to limit detrimental inflammatory responses and promote metabolic homeostasis both locally and systemically [27]. Both human and animal studies have shown that helminths may adopt an immune evasion strategy, wherein helminth infection may decrease systemic inflammation and consequently the progression of inflammatory diseases, including T2DM [28–30], most likely by modulating host immune responses. Anthelmintic treatment might ameliorate this helminth-associated protective effect in T2DM and other metabolic syndromes [28, 31]. Hence, the helminth infection–T2DM interface needed to be explored further.
Vascular malfunction plays key role in the pathogenesis of T2DM, and the main characteristic features of diabetic complications include dysregulated angiogenesis and endothelial activation. Abnormal angiogenesis is a distinctive pathological feature of the microvasculature in T2DM [32]. Diabetes affects the retina by excessive formation of premature blood vessels and impaired wound healing in the small retinal blood vessels [32], findings that support the use of angiogenic inhibitors in the treatment of diabetic retinopathy [33, 34]. Helminth infection on top of T2DM is associated with decreased levels of VEGF-A, -C, and -D and their endogenous receptors.
The vascular endothelial growth factor receptors are one of the most important signaling pathways that regulate angiogenesis [35]. Hyperglycemia affects the expression of VEGF and its receptors VEGF-R1 and VEGF-R2 [36]. VEGF receptors mediate various cellular functions. VEGFs can bind to 3 distinct receptors: previous literature suggested that VEGF-R1 plays a role in physiological and pathological neovascularization, whereas VEGF-R2 and -R3 drive angiogenesis [37]. Indeed, previous studies have shown that helminths might protect against insulin resistance in T2DM [29, 38]. We and others have shown that those with T2DM and coincident Ss infection had lower insulin and blood glucose levels than did those with T2DM alone [21, 38]. The present study has demonstrated that Ss infection also has a significant impact on the levels of VEGF-A, VEGF-C, VEGF-D, VEGF-R1, VEGF-R2, and VEGF-R3 in individuals with coexistent T2DM. These results imply that helminths might limit the proangiogenic process. Anthelmintic therapy of Ss infection, not surprisingly, appears to cause reversion of these levels to those seen in persons with T2DM alone. The mechanism by which helminth infection modulates these factors warrants further studies.
AGE plays an important role in chronic inflammatory conditions [39]. AGE binds to multiple families of ligands, namely RAGE, the S100/calgranulin family, and HMGB-1 [40]. Reactive oxygen species induced by hyperglycemia trigger various pathways of diabetic tissue damage, increase intracellular AGE formation, increase RAGE levels [41], and results in the release of intracellular calcium binding molecules, the S100 calgranulins [42] and HMGB-1 [43]. These molecules trigger a proinflammatory cascade. Highly enhanced levels of these receptors and their ligands appear to contribute to some of the complications of T2DM [44] and have been implicated in mediating insulin resistance [20].
Several experimental and epidemiological studies reported that helminths protect against metabolic syndromes, T2DM, and obesity [1, 21, 38, 45–47]. Following definitive anthelmintic therapy, significant reversion of these parameters has been observed [21, 31]. Our study is not a randomized controlled trial; thus, the effect of increased angiogenic factors after the anthelmintic treatment can be contributed to by either suppression of Ss infection, the anthelmintic drugs (ivermectin and albendazole), or both. Our data imply that Ss infection might have a role in delaying or preventing the onset of diabetic complications by reducing the systemic levels of AGE and their ligands sRAGE, S100A12, and HMGB-1, glycation, and tissue AGEs.
In summary, our data show the beneficial effects of helminth on individuals with T2DM associated with angiogenesis, lymphangiogenesis, and vascular dysfunction. These benefits may occur through the modulation of a VEGF/VEGF-R interaction along with AGE/RAGE/ligand interaction. A more detailed characterization of the immunomodulatory mechanisms of helminths could endorse more specific treatment methods against T2DM. This could be helpful for management of complications and thus reduce the rate of complications as well as the economic influence of disease on the patients and the public.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure 1. Circulating levels of angiogenic factors and RAGE ligands in Ss + and Post-T individuals. (A) Plasma levels of Plasma levels of VEGF-A, VEGF-C, VEGF-D, Angio-I, Angio-2, VEGF-R1, VEGF-R2, VEGF-R3 and Angio-2/ Angio-I ratio in T2DM with Ss + pre-treatment [Baseline, n = 60] and 6 months following treatment [Treated, n = 60] were measured. (B) Plasma levels of AGE, sRAGE, S100A12 and HMGB-1 in T2DM with Ss + pre-treatment [Baseline] [n = 60] and 6 months following treatment [Treated] [n = 60] were measured. The levels of the angiogenic factors for each subject is shown as spaghetti plots. Dark line with open circle represents the baseline and dotted line with closed circle represents the treated individuals.
Supplementary Figure 2. No significant differences in the circulating levels of angiogenic factors and RAGE ligands between Strongyloides stercoralis–uninfected (Ss–) and posttreatment (Post-T) individuals. A, Plasma levels of vascular endothelial growth factor (VEGF)–A, VEGF-C, VEGF-D, angiopoietin (Ang) 1, and Ang-2 in type 2 diabetes mellitus (T2DM) in Ss– (n = 58) and Post-T (n = 60) individuals. B, Plasma levels of VEGF-R1, -R2, -R3, and Ang-2/Ang-1 ratio in T2DM in Ss– (n = 58) and Post-T (n = 60) individuals. C, Plasma levels of advanced glycation end products (AGEs), soluble receptor for advanced glycation end product (sRAGE), S100A12, and high mobility group box 1 (HMGB-1) in T2DM in Ss– (n = 58) and Post-T (n = 60) individuals. Each dot represents an individual subject with the bar representing the geometric mean. Mann–Whitney U test was performed to calculate the P values.
Supplementary Figure 3. Diminished circulating levels of angiogenic factors and RAGE ligands in Ss + individuals. (A) Plasma levels of VEGF-A, VEGF-C, VEGF-D, Angio-I and Angio-2 T2DM with Ss + (n = 60) and without Ss- (n = 58) individuals. (B) Plasma levels of VEGF-R1, VEGF-R2, VEGF-R3 and Angio-2/ Angio-1 ratio in T2DM with Ss + (n = 60) and without Ss- (n = 58) individuals. (C) (A) Plasma levels of AGE, sRAGE, S100A12 and HMGB-1 in T2DM with Ss + (n = 60) and without Ss- (n = 58) individuals. Within each box, horizontal line represents median values, the black box contains the 25th to 75th percentiles of dataset. The black whiskers mark the 5th and 95th percentiles. Mann– Whitney U-test with Holms correction for multiple comparisons were done by p-values are multiplied by the number of parameters.
Notes
Acknowledgments. We thank Dr M. Satiswaran, B. Suganthi, and P. Balakrishnan for valuable assistance in collecting the clinical data for this study. We thank the staff of the Department of Epidemiology, National Institute for Research in Tuberculosis, for valuable assistance in recruiting the patients for this study.
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, US National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tracey EF, McDermott RA, McDonald MI. Do worms protect against the metabolic syndrome? A systematic review and meta-analysis. Diabetes Res Clin Pract 2016; 120:209–20. [DOI] [PubMed] [Google Scholar]
- 2. Guigas B, Molofsky AB. A worm of one’s own: how helminths modulate host adipose tissue function and metabolism. Trends Parasitol 2015; 31:435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tahergorabi Z, Khazaei M. Imbalance of angiogenesis in diabetic complications: the mechanisms. Int J Prev Med 2012; 3:827–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 2009; 10:165–77. [DOI] [PubMed] [Google Scholar]
- 5. Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett 2013; 328:18–26. [DOI] [PubMed] [Google Scholar]
- 6. Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells 2019; 8:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature 2001; 411:355–65. [DOI] [PubMed] [Google Scholar]
- 8. Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev 2003; 23:117–45. [DOI] [PubMed] [Google Scholar]
- 9. Khazaei M, Fallahzadeh AR, Sharifi MR, Afsharmoghaddam N, Javanmard SH, Salehi E. Effects of diabetes on myocardial capillary density and serum angiogenesis biomarkers in male rats. Clinics (Sao Paulo) 2011; 66:1419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fallahzadeh AR, Khazaei M, Sharifi MR. Restoration of angiogenesis by enalapril in diabetic hindlimb ischemic rats. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2011; 155:137–42. [DOI] [PubMed] [Google Scholar]
- 11. Lim HS, Lip GY, Blann AD. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis 2005; 180:113–8. [DOI] [PubMed] [Google Scholar]
- 12. Maisonpierre PC, Suri C, Jones PF, et al. . Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997; 277:55–60. [DOI] [PubMed] [Google Scholar]
- 13. Kakizawa H, Itoh M, Itoh Y, et al. . The relationship between glycemic control and plasma vascular endothelial growth factor and endothelin-1 concentration in diabetic patients. Metabolism 2004; 53:550–5. [DOI] [PubMed] [Google Scholar]
- 14. Tsai YC, Lee CS, Chiu YW, et al. . Angiopoietin-2, renal deterioration, major adverse cardiovascular events and all-cause mortality in patients with diabetic nephropathy. Kidney Blood Press Res 2018; 43:545–54. [DOI] [PubMed] [Google Scholar]
- 15. Kay AM, Simpson CL, Stewart JA Jr. The role of AGE/RAGE signaling in diabetes-mediated vascular calcification. J Diabetes Res 2016; 2016:6809703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soman S, Raju R, Sandhya VK, et al. . A multicellular signal transduction network of AGE/RAGE signaling. J Cell Commun Signal 2013; 7:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dorenkamp M, Müller JP, Shanmuganathan KS, et al. . Hyperglycaemia-induced methylglyoxal accumulation potentiates VEGF resistance of diabetic monocytes through the aberrant activation of tyrosine phosphatase SHP-2/SRC kinase signalling axis. Sci Rep 2018; 8:14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daffu G, del Pozo CH, O’Shea KM, Ananthakrishnan R, Ramasamy R, Schmidt AM. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int J Mol Sci 2013; 14:19891–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Zhong J, Zhang X, et al. . The role of HMGB1 in the pathogenesis of type 2 diabetes. J Diabetes Res 2016; 2016:2543268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol 2014; 18:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rajamanickam A, Munisankar S, Bhootra Y, et al. . Metabolic consequences of concomitant Strongyloides stercoralis infection in patients with type 2 diabetes mellitus. Clin Infect Dis 2019; 69:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rajamanickam A, Munisankar S, Dolla C, et al. . Helminth infection modulates systemic pro-inflammatory cytokines and chemokines implicated in type 2 diabetes mellitus pathogenesis. PLoS Negl Trop Dis 2020; 14:e0008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deepa M, Pradeepa R, Rema M, et al. . The Chennai Urban Rural Epidemiology Study (CURES)—study design and methodology (urban component) (CURES-I). J Assoc Physicians India 2003; 51:863–70. [PubMed] [Google Scholar]
- 24. Bisoffi Z, Buonfrate D, Sequi M, et al. . Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 2014; 8:e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buonfrate D, Formenti F, Perandin F, Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect 2015; 21:543–52. [DOI] [PubMed] [Google Scholar]
- 26. Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol 2020; 11:1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mishra PK, Palma M, Bleich D, Loke P, Gause WC. Systemic impact of intestinal helminth infections. Mucosal Immunol 2014; 7:753–62. [DOI] [PubMed] [Google Scholar]
- 28. Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis 2014; 14:1150–62. [DOI] [PubMed] [Google Scholar]
- 29. Wiria AE, Sartono E, Supali T, Yazdanbakhsh M. Helminth infections, type-2 immune response, and metabolic syndrome. PLoS Pathog 2014; 10:e1004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tahapary DL, de Ruiter K, Martin I, et al. . Helminth infections and type 2 diabetes: a cluster-randomized placebo controlled SUGARSPIN trial in Nangapanda, Flores, Indonesia. BMC Infect Dis 2015; 15:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tahapary DL, de Ruiter K, Martin I, et al. . Effect of anthelmintic treatment on leptin, adiponectin and leptin to adiponectin ratio: a randomized-controlled trial. Nutr Diabetes 2017; 7:e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng R, Ma JX. Angiogenesis in diabetes and obesity. Rev Endocr Metab Disord 2015; 16:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar A, Bade G, Trivedi A, Jyotsna VP, Talwar A. Postural variation of pulmonary diffusing capacity as a marker of lung microangiopathy in Indian patients with type 2 diabetes mellitus. Indian J Endocrinol Metab 2016; 20:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev 2014; CD007419. [DOI] [PubMed] [Google Scholar]
- 35. Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995; 376:66–70. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Q, Fang W, Ma L, Wang ZD, Yang YM, Lu YQ. VEGF levels in plasma in relation to metabolic control, inflammation, and microvascular complications in type-2 diabetes: a cohort study. Medicine (Baltimore) 2018; 97:e0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ho VC, Duan LJ, Cronin C, Liang BT, Fong GH. Elevated vascular endothelial growth factor receptor-2 abundance contributes to increased angiogenesis in vascular endothelial growth factor receptor-1-deficient mice. Circulation 2012; 126:741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moyat M, Coakley G, Harris NL. The interplay of type 2 immunity, helminth infection and the microbiota in regulating metabolism. Clin Transl Immunology 2019; 8:e01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamamoto Y, Yamamoto H. RAGE-mediated inflammation, type 2 diabetes, and diabetic vascular complication. Front Endocrinol (Lausanne) 2013; 4:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 2001; 108:949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yan SF, Ramasamy R, Schmidt AM. Receptor for AGE (RAGE) and its ligands-cast into leading roles in diabetes and the inflammatory response. J Mol Med (Berl) 2009; 87:235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Donato R. RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Curr Mol Med 2007; 7:711–24. [DOI] [PubMed] [Google Scholar]
- 43. Yan XX, Lu L, Peng WH, et al. . Increased serum HMGB1 level is associated with coronary artery disease in nondiabetic and type 2 diabetic patients. Atherosclerosis 2009; 205:544–8. [DOI] [PubMed] [Google Scholar]
- 44. Park L, Raman KG, Lee KJ, et al. . Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 1998; 4:1025–31. [DOI] [PubMed] [Google Scholar]
- 45. Chen Y, Lu J, Huang Y, et al. . Association of previous schistosome infection with diabetes and metabolic syndrome: a cross-sectional study in rural China. J Clin Endocrinol Metab 2013; 98:E283–7. [DOI] [PubMed] [Google Scholar]
- 46. Shen SW, Lu Y, Li F, et al. . The potential long-term effect of previous schistosome infection reduces the risk of metabolic syndrome among Chinese men. Parasite Immunol 2015; 37:333–9. [DOI] [PubMed] [Google Scholar]
- 47. Aravindhan V, Mohan V, Surendar J, et al. . Decreased prevalence of lymphatic filariasis among diabetic subjects associated with a diminished pro-inflammatory cytokine response (CURES 83). PLoS Negl Trop Dis 2010; 4:e707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.