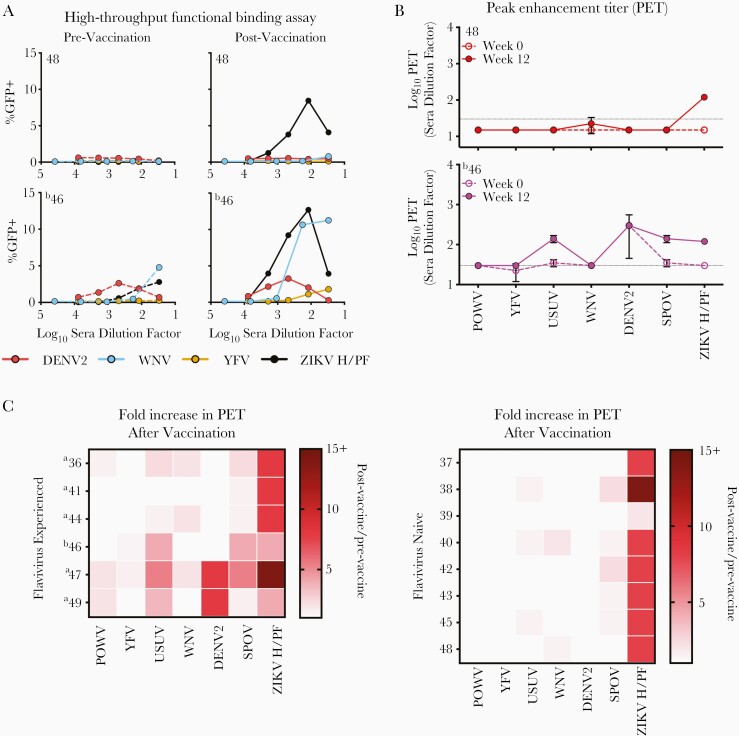
Figure 2.
Cross-reactive antibody binding of vaccine-induced antibody responses in humans. Antibody-dependent enhancement (ADE) assays were performed as a sensitive measure of antibody binding to infectious virions in solution. Serum was serially diluted, incubated with indicated reporter virus particles (RVPs), and used to infect semipermissive FcγR-expressing K562 cells. Enhancement was scored by green fluorescent protein (GFP) expression and measured after 36–48 hours using flow cytometry. (A) Representative data using pre- and postvaccination sera from 2 VRC5283 vaccine recipients are shown. (B) The serum dilution corresponding to the highest level of signal (peak enhancement titer [PET]) was determined. The PET was considered positive if ≥3-fold above the background level of infection for each RVP in the absence of antibody. Prevaccination and 12 weeks postfirst vaccine dose serum samples were studied. Dashed and solid lines represent the average PET obtained with pre- and postvaccination serum, respectively. The horizontal dotted line represents the limit of detection (LOD) of the assay. Any measurement below the LOD was assigned a value of half the LOD. Error bars represent the range of 2 independent experiments. (C) The change in PET occurring after vaccination of flavivirus experienced (left panel) or flavivirus naive (right panel) recipients was calculated and displayed as the fold-difference, or quotient of the postvaccination PET by the prevaccination PET, on a heat map. For samples with no detectable binding activity, the half value of the number assigned to the LOD was used in these calculations (a value of 15). Smaller PET fold-differences are indicated in lighter red; larger differences are indicated in dark red. aYellow fever virus (YFV) vaccine immunization history; bpositive for dengue virus (DENV) IgG at time of vaccination.