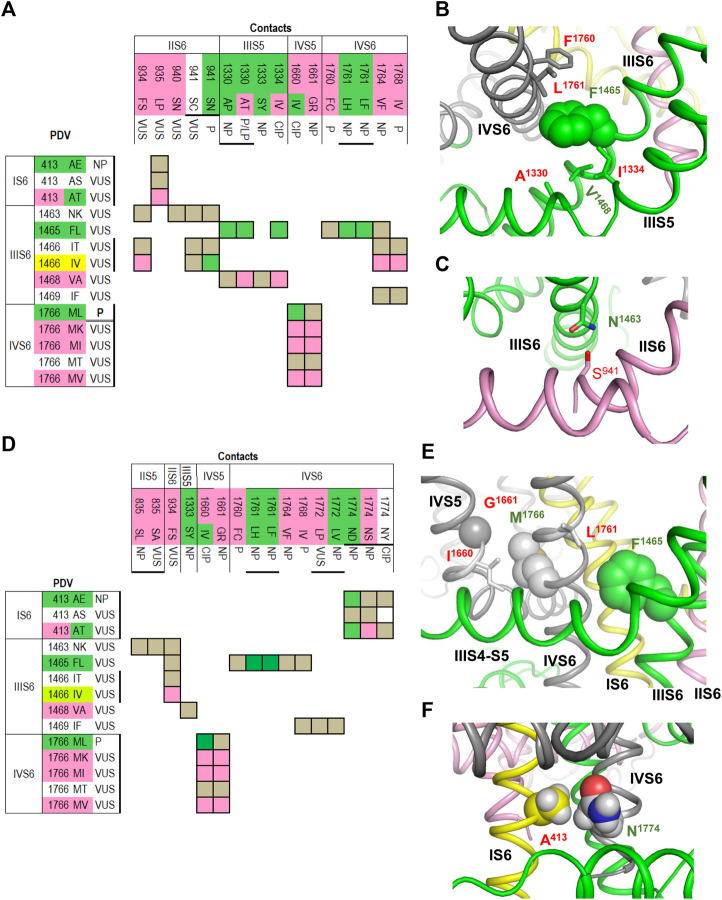
FIGURE 4.
Open and closed conformations of PD. (A–C) Open PD model. (A) Matrix of contact variants shows only those PDVs and their contacts, which are unique in the open PD model. Most of coupled variants are associated with either BrS1 or LQT3. (B) Hydrophobic contacts L1761:F1465:F1760 and A1330:V1468:I1334 stabilize, respectively, mutual disposition of helices IIIS6/IVS6 and IIIS5/IIIS6. (C) H-bond S941:N1463 stabilizes mutual disposition of helices IIS6/IIIS6. (D–F) Closed PD model. (D) Matrix of contact variants shows only those PDV and their contacts, which are unique in the closed-PD model. (E) Mutual disposition of helices IIIS6 and IVS6 in the closed PD is stabilized by hydrophobic contacts F1465:L1761 with side chain conformations different from those in the open-PD model. In coupled LQT3-associated variants, substitutions F1465L or L1761F/H would destabilize the closed PD. Mutual disposition of helices IVS5/IVS6 is stabilized by a hydrophobic contact M1761:I1660 and a knob-into-hole contact M1761:G1661. (F) Mutual disposition of helices IS6/IVS6 is stabilized by a tight hydrophobic contact between A413 and methylene group of N1774. Replacement of any contact partner with a large residue would destabilize the closed PD, a likely cause of LQT3 associated with these mutations.