Abstract
Anatomic models are important in medical education and pre-operative planning as they help students or doctors prepare for real scenarios in a risk-free way. Several experimental anatomic models were made with additive manufacturing techniques to improve geometric, radiological, or mechanical realism. However, reproducing the mechanical behavior of soft tissues remains a challenge. To solve this problem, multi-material structuring of soft and hard materials was proposed in this study, and a three-dimensional (3D) printer was built to make such structuring possible. The printer relies on extrusion to deposit certain thermoplastic and silicone rubber materials. Various objects were successfully printed for testing the feasibility of geometric features such as thin walls, infill structuring, overhangs, and multi-material interfaces. Finally, a small medical image-based ribcage model was printed as a proof of concept for anatomic model printing. The features enabled by this printer offer a promising outlook on mimicking the mechanical properties of various soft tissues.
Keywords: Silicone 3D printing, Multi-material 3D printing, Anatomic models, Soft tissues
1. Introduction
1.1. Anatomic models
In medical practice and education, anatomic models are provided through using human donors, animal models, or artificial technical solutions that range from hand-crafted training models to mass-produced commercial products. In the former case of using real biological tissues, progress is often hindered by the lack of available human donors, strict regulations regarding animal and human testing, and problems in experiment repeatability due to the anatomical uniqueness of every human or animal specimen[1,2]. Using advanced artificial anatomical models has the potential to ease these problems, especially in case of anatomy or surgical education, pre-operative planning, or development of novel medical devices[3,4]. Studies show that the use of physical anatomic models improves medical education from various aspects due to the additional haptic and spatial information students could not receive through books or screen visualizations[5-7]. In the surgical domain, anatomical models can aid the planning of complicated surgeries in a wide range of surgical specialties, since rehearsing the steps of the operation on a patient-specific model can reveal upcoming intra-operative complications[2,8-11]. This can significantly reduce the risk and duration of certain operations, which may result in the lower risk of complication and higher patient satisfaction[12]. Moreover, patient-specific models help the development of various customized implants and other medical instruments[12-14].
Traditionally, artificial anatomical models are mass-produced through casting or molding techniques, often based on population-averaged geometries[3,13]. The materials used in such models are usually different hard and soft polymers, such as thermoplastics, waxes, or rubbers. With casting and molding techniques, the mechanical properties of various represented tissues can be matched mainly through material selection as these traditional technologies produce fully dense parts. This level of matching is often sufficient for certain mass-produced educational models[6], but the requirements of medical product development and testing, as well as preoperative planning may benefit from a better mechanical fidelity[3,14-17].
1.2. Additive manufacturing (AM) of anatomic models
AM, also called three-dimensional (3D) printing, has become an increasingly influential group of technologies in the field of anatomic models and other medically relevant areas in recent years[12,18,19]. Achieving better geometric and mechanical fidelity is possible with the combination of medical imaging technologies and AM[20]. For polymeric materials, the two dominant groups of AM techniques are based on photopolymerization and on extrusion[3]. In an extrusion-based technique called fused filament fabrication (FFF), a thermoplastic filament is pushed into a heated extruder, and deposited through a nozzle[21]. This is mostly used for bone modeling and mold making in the field of anatomic models[3,9]. FFF is cheaper than most other AM technologies due to its relative simplicity and fierce competition between several manufacturers. These systems can process a large variety of hard thermoplastic filaments but are limited in their ability to handle soft materials. A large proportion of available medical image-based anatomic models are made of hard plastic using FFF[20].
Liquid photopolymers can be deposited and solidified in small droplets via material jetting, which is called inkjet printing (IJP). Among others, it has been used to create surgical training models of aortic aneurysms, kidney tumors, skulls and fetuses[15,22,23]. IJP can also use multi-colored inks to make full-color objects[9,20] and even use multiple hard and soft materials in a single print job[14,16,17,24,25]. The deposited droplets can be understood as voxels. Given the proper printhead, this allows multiple voxels of multiple materials to be deposited simultaneously[26]. While changing or mixing materials in a single droplet generator unit is difficult, IJP can easily achieve anisotropic properties by creating inclusions of various materials[16,17,27], or even seemingly gradient composition change[28]. However, IJP is limited in creating hollow and completely closed cavities because droplets need support underneath them. Therefore, internal structuring is only possible if the support material can be washed or cut out after printing without damaging the printed object. From the standpoint of anatomic models, a relevant IJP printer on the market is the J750 Digital Anatomy Printer by Stratasys Ltd. (Eden Pairie, MI)[24,29]. This offers an outstanding performance concerning geometric representation and the number of materials and colors used, including soft materials[30,31]. However, the mechanical realism of soft tissue representing materials is still criticized[15].
Using soft materials is an intensely researched direction of AM[32]. Besides IJP, thermoset, photoreactive or chemically cured materials like certain silicones, resins or hydrogels may be deposited through extrusion as well, which is also called direct ink writing (DIW)[3,33-35]. This is used to print models of various soft structures[3,36-38]. Most of these operate with pressurized material reservoirs with controllable valves or syringe extruders to deposit soft materials. The rheological properties of the printed material, such as viscosity or thixotropy, are decisive for maintaining the shape of the printed object. Creating closed air inclusions is theoretically possible with FFF and certain DIW techniques[20].
Silicone rubbers offer a range of mechanical properties that may be ideal to represent soft tissues in anatomic models[33]. Certain silicone AM (SAM) technologies are already being applied to anatomical models in some research endeavors and early-stage commercial services[33,39-47]. These are summarized in Table 1. The collaboration of Wacker Chemie AG (Munich, Germany) with ACEO (Burghausen, Germany) led to a droplet-based silicone printing technology, which relies on curing each layer of silicone with UV light, in a similar fashion to IJP[39,48]. Dow Inc. (Midland, MI) and German RepRap GmbH (Feldkirchen, Germany) created an extrusion-based technology called Liquid AM (LAM) which deposits silicone with extrusion and cures it layer-wise using a heat source[40,49]. Another SAM process is developed by Spectroplast AG (Zürich, Switzerland), a spinoff company of ETH Zürich. This method uses layer-wise photopolymerization in a liquid silicone bath[43]. Another method called Picsima by Fripp Design Ltd. (Rotherham, UK) represents a different bath-based printing approach, namely extruding the catalyst component of a two-part silicone into a bath of the base component[41]. SAM may also utilize a non-planar coordinate system. Coulter et al. developed a printing method specialized on rotating printing surfaces, which offers unique advantages in realizing certain geometries[44,45]. Despite the promising development that these technologies represent, almost all focus on single-material printing. Therefore, the capabilities to tune mechanical properties are limited to realizing porous structures with internal cavities[32].
Table 1.
Summary of relevant commercial soft material printing technologies
| Group name | Process name | Principle | Material |
|---|---|---|---|
| Stratasys Ltd. | J750 | Droplet jetting | Photopolymers |
| Wacker Chemie AG | ACEO | Droplet jetting | Silicone rubbers |
| Dow Inc./GermanRepRap GmbH | LAM | Extrusion | Silicone rubbers |
| Fripp Design Ltd. | Picsima | Extrusion | Silicone rubbers |
| Spectroplast AG | Spectroplast | Vat photo-polymerization | Silicone rubbers |
1.3. Problems in mechanical realism
These AM technologies (IJP, FFF, and DIW) are highly applicable to create personalized anatomic models that are geometrically unique[3]. However, geometric or color fidelity alone do not satisfy all possible needs of medical device development, surgical education, or preoperative planning. For more advanced applications, models should behave realistically under physical manipulation with hands or surgical instruments[20]. To achieve such surgical realism, the materials used to represent various biological tissues need to have similar mechanical properties to the tissues, such as density, elastic modulus, hardness, tensile strength, or viscoelasticity[15-17]. While matching hard tissues like bone with AM is already a mature field, there are still many unsolved problems regarding soft tissues[20]. Most biological tissues – unlike technical materials – exhibit multi-level hierarchic structures of various functional building blocks, which often results in anisotropic and viscoelastic mechanical properties[15,50]. This behavior could be approximated with soft-hard multi-material structures[14,16,17], but to date, there are no AM technologies available that can approximate a multitude of tissues[15]. Therefore, two major areas for improvement could be printing both hard and soft materials simultaneously, and tuning local mechanical properties through multi-material structuring. These should happen simultaneously to produce high quality anatomic models that resemble real tissues from a mechanical standpoint[15].
1.4. Research aims
Combining extrusion-based AM technologies such as FFF and DIW may be helpful for making more realistic anatomic models. While using FFF to produce the whole model is ineffective regarding mechanical realism, thermoplastics may be used as fiber reinforcement if printed into a softer matrix material, like a silicone rubber that is deposited by a DIW printhead. Such a concept may also work with having both the soft matrix and a harder reinforcement being deposited through DIW[51]. In any case, this strategy would allow the hardening, toughening (further referred to as “up-tuning”) of bulk mechanical properties compared to the original matrix material. Since both FFF and extrusion-based DIW can print closed and empty cavities, the weakening, and softening (further referred to as “down-tuning”) of mechanical properties would also be possible[15].
Therefore, the main aim of this research was to design, build and test a 3D printer based on the concept of combining hard and soft materials for printing more realistic anatomic models. As a proof of concept, the printer should be capable of printing at least one soft and one hard material, and thus achieve both up-tuning and down-tuning to influence mechanical properties. Moreover, the printer should also realize thin-walled structures and closed internal cavities with the soft material since these are relevant features in anatomic models. In this study, a 3D printer with these features was built, and its abilities were evaluated through qualitative analysis of various printed proof-of-concept objects, including a small ribcage model based on a medical image. The applicability of the system in the field of anatomic models and the future direction of research are also discussed.
2. Materials and methods
2.1. Technology definition
The design process of this novel AM system started with a comparison of various AM technologies and their specifications, as clarifying differences is critical for choosing the right printing concept. The fact that IJP, DIW, and FFF can handle different materials in the same print is a required feature to produce multi-material structures. Other technologies based on material jetting or vat photopolymerization, such as binder jetting (BJ), stereolithography (SLA), and digital light processing (DLP) all use a single-material bath (or “vat”) of liquid resin or powder[21]. This prevents multi-material printing, and the creation of closed air inclusions. For IJP, DIW, and FFF, changing materials simply requires switching to a different filament, cartridge, or printhead. Mimicking the macroscopic mechanical properties of biological tissues through up- and down-tuning requires printing both soft and hard materials. Extrusion is the preferred method to create closed internal cavities and support structures, if needed. The mentioned technologies are compared considering our construction preferences in Table 2. Further descriptions and schematics of these technologies are available in other literature[21,32].
Table 2.
A survey of features considering various additive manufacturing technologies
| Technology | Principle | Soft materials | Multi- material | Material deposition | Support structures | Closed cavities |
|---|---|---|---|---|---|---|
| SLS | Powder bed fusion | Limited | No | Spreading | Powder or printed | No |
| BJ | Material jetting | Limited | No | Spreading and droplets | Powder or printed | No |
| IJP | Material jetting | Yes | Yes | Droplets | Printed | Limited |
| SLA | Vat photo-polymerization | Limited | No | Spreading | Liquid or printed | No |
| DLP | Vat photo-polymerization | Limited | No | Spreading | Liquid or printed | No |
| FFF | Extrusion | Limited | Yes | Heated extrusion | Printed | Yes |
| DIW | Extrusion | Yes | Yes | Extrusion or droplets | Liquid or printed | Yes |
| *Ideal for anatomic models | Extrusion | Yes | Yes | Extrusion | Printed | Yes |
An imaginary technology which we found ideal for anatomic models if mechanical realism is desirable.
Concerning the printing material, some silicone rubbers exhibit ideal mechanical properties to mimic various soft tissues. This also makes them a popular casting material for certain anatomic models, where a casting mold of the desired anatomy is first printed with FFF or SLA, and then filled with a two-component addition-cured silicone rubber[7,9]. However, in multi-material printing, the adhesion of the various printing materials is important, unlike in casting. Certain single-component condensation-cured silicone rubbers may exhibit an adhesive behavior to some thermoplastic polymer materials, which makes these a promising combination in a multi-material printing scenario. Therefore, the printer should employ an FFF printhead for printing thermoplastics, and a continuous extrusion-based DIW printhead to print single-component silicone rubbers.
2.2. Printer system
For an extrusion-based DIW printhead, various extrusion mechanisms, such as syringes, peristaltic pumps and screw extruders, are available. However, for high-viscosity and high-precision applications, screw extruders were preferred. The final choice fell on a Vipro-HEAD 3/3 two-component printhead by Viscotec GmbH (Töging am Inn, Germany)[52], which enables processing either one or two single-component silicones, or a two-component silicone. In this study, only one single-component silicone rubber was used.
Regarding printer mechanics from the standpoint of printing soft and flexible materials, it is important that the building platform only moves in the axis of the building direction (usually labeled “Z”), so that it does not shake the printed objects horizontally during printing. The printer kinematics which fulfills this criterion are the so-called XY-Core and the Delta kinematics. Hardware and software formed another important aspect in the component selection. An FFF printer which does not only apply Delta or XY-Core kinematics, but also employs a control board that is open-source and easily extended with the chosen Viscotec extruder was considered highly desirable.
Finally, a Railcore II 300 ZL open-source FFF 3D printer system[53] was chosen and modified (Figure 1). On this printer, the original E3D V6 FFF printhead was extended with the Viscotec Vipro-HEAD 3/3 (Figure 2)[52]. Silicones and other high-viscosity materials can be fed into this screw extruder with pressurized air up to 6 bars, from 55 mL cartridges, which are also mounted on the printhead. If necessary, these can be moved to the frame, which removes their volume and mass limitations, enabling a large material supply to the printhead given that the feed pressure is sufficient. The silicone printing nozzle is connected to the outlet of the extruder through a Luer-thread and is secured against unscrewing with a retainer part. These white Luer-adapters and retainers were custom-made for the extruder (Figure 2A). A nozzle with 0.33 mm outlet diameter was selected for silicone extrusion. The original E3D V6 FFF printhead on the other side of the carriage (Figure 2B) is capable of melting and depositing thermoplastic filaments through a 0.4 mm diameter nozzle.
Figure 1.
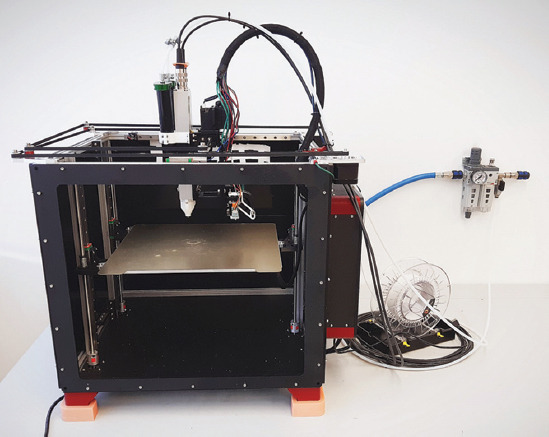
The modified Railcore II 300 ZL printer, extended with a Viscotec Vipro-HEAD 3/3 extruder.
Figure 2.

The Viscotec Vipro-HEAD 3/3 extruder with custom Luer-compatible endpieces (A), and the original E3D V6 filament extruder on the opposite side of the printhead carriage (B).
The printer is controlled by the Duet 2 Wi-Fi control electronics, extended with a Duex 5 extension board, operating with RepRap v1.18 firmware[54]. The system can be connected to a personal computer (PC) through a Wi-Fi network, and print jobs can be started through the Duet Web Interface, which is accessible through an internet browser running on the PC. The general printer configuration, including the printhead definitions and dosing calibration settings are done by modifying a file stored on the Duet 2 Wi-Fi board. The slicing software used to generate G-codes for printing objects is Prusa Slicer (version 2.1), an open-source slicer originally made for filament-based printers[55]. The user can easily define a multi-material printhead and generate G-codes for multi-material FFF-DIW print jobs. No post-processing of the generated G-code files is necessary, and an extrusion correction factor can also be set to fine-tune dosing accuracy, when necessary. To start a print job, the generated G-code files must be uploaded to the Duet 2 Wi-Fi board through the Duet Web Interface. This way, the printer is likely also compatible with other popular slicing software, such as Cura or Simplify3D.
2.3. Materials
The selected silicone material is a high-viscosity single-component condensation-crosslinking liquid silicone rubber called Elkem AMSil 20101 (Elkem Silicones, Oslo, Norway), which was used with the Viscotec DIW printhead. This material is intended for cold extrusion; therefore, no heating or other means of energy input is required during printing. Moreover, a 1.75 mm diameter poly-lactic acid (PLA) filament from Fillamentum Manufacturing (Hulín, Czech Republic) was used with the E3D V6 FFF printhead in case of prints that demonstrate the multi-material capabilities. PLA was chosen as it is an easily accessible and popular FFF material.
2.4. Printing tests
For accurate dosing, the silicone printhead was calibrated for the chosen material using a KERN PES 42002M scale (Kern & Sohn GmbH, Balingen, Germany). After this, 15 × 15 × 10 mm silicone blocks were printed with various speeds and layer thicknesses to find reasonable settings for further printing tests. The integrity of the printed cubes was qualitatively evaluated by observing them and then slicing them with a blade to see if there are any internal faults (Figure 3).
Figure 3.

A 15 × 15 × 10 mm test block that was printed after calibration and cut in half after printing.
Based on the calibration prints, a printing speed of 15 mm/s and a layer thickness of 0.3 mm were chosen for further trials. Furthermore, every printed object was left on the building platform untouched for 24 h to ensure sufficient crosslinking before any manipulation or inspection. After calibration, the system’s ability to print silicone objects with closed internal cavities, infill structuring and thin walls as well as to combine silicone DIW and thermoplastic FFF was assessed by conducting six printing tests:
In the first test, a thin-walled shell was printed based on the same 15 × 15 × 10 mm cuboid that was used for the calibrations. In this case, only two lines of outer contour were used, resulting in approximately 0.7 mm shell wall thickness (Figure 4). This is relevant to anatomic models in case of printing vessels or membranes, which feature thin walls.
The second test involved a silicone block of the same dimensions as in the first test, but with 40% volume fraction gyroid infill structuring to simulate down-tuning (Figure 5).
In the third test, a 50% downscaled version of a human bladder was printed based on a 3D model segmented from a computed tomography (CT) image earlier. To test the feasibility of such a large internal cavity with overhanging areas, no support was used inside. The envelope dimensions of this downscaled bladder were approximately 35 × 30 × 25 mm, and the wall thickness was changing between 1.5 and 2 mm (Figure 6).
The fourth test involved a pair 15 × 15 × 3 mm square silicone and PLA multi-material chips on top of each other. The silicone was printed on top of the PLA to simulate a situation of printing hard plastic support under a silicone structure (Figure 7A).
In the fifth test, the same model was used as in the fourth test, but now the PLA was printed on top of the silicone to simulate laying the filament as an inclusion into the silicone matrix (Figure 7B).
The sixth test was planned to give some synthesis of the features investigated through the previous tests. The ribcage and the surrounding soft tissues were segmented from the CT image of a newborn using 3D-Slicer, and the model was printed (Figure 8). The ribs and the support structures were printed from PLA and the surrounding soft tissue was printed from silicone. To mimic bone structure, the ribs were printed with a 30% gyroid infill, while 80% infill was used for the soft tissue.
Figure 4.
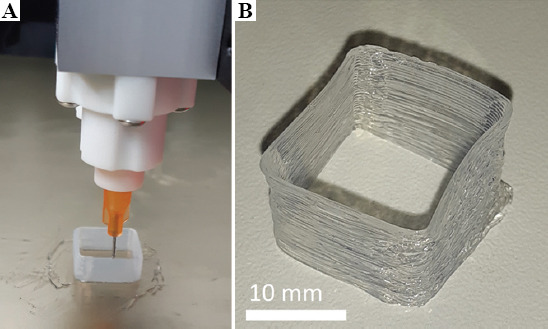
Thin-walled silicone rubber shell during (A) and after printing (B).
Figure 5.
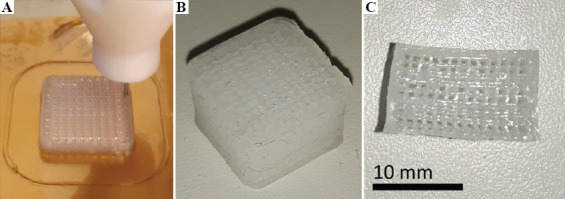
Silicone block with 40% volume fraction gyroid infill during (A) and after printing (B), and after slicing with a blade (C) to reveal the internal structure.
Figure 6.
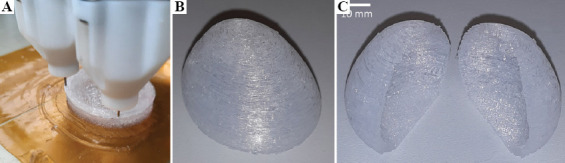
A downscaled human bladder with no internal support during (A) and after printing (B), and after slicing with a blade (C) to reveal the internal cavity.
Figure 7.
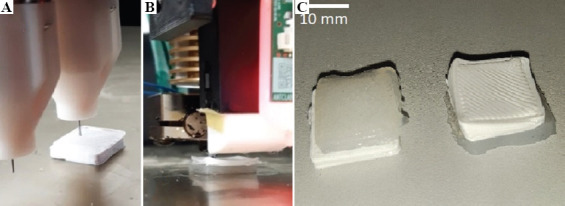
Printing silicone rubber on top of PLA (A), then PLA on top of silicone rubber (B), and the resulting multi-material chips from both tests (C) .
3. Results
All test objects were successfully printed. The thin-walled shell of the first test (Figure 4) did not collapse during or after printing, despite having only 0.7 mm wall thickness.
The 40% gyroid block of the second test (Figure 5) was cut in half with a blade after crosslinking to reveal the internal structure (Figure 5C).
The downscaled bladder in the third test (Figure 6) had minor material integrity errors at the top due to the lack of internal support, but the external geometry stayed intact. This bladder was also cut in half after printing to reveal the internal cavity (Figure 6C).
The multi-material chips from the fourth (Figure 7C) and fifth (Figure 7B) tests were also printable. Moreover, in case of the fifth test, the PLA top was deformed, presumably due to printing on a soft and unstable silicone surface. After printing, the adhesion between the silicone and the PLA in the multi-material chips was evaluated by trying to manually separate the materials. The silicone was considered adhesive enough to resist this manual peeling, since the bulk silicone material was damaged before the interface.
After seeing the success of the five previous tests, the ribcage model of the sixth test was printed to demonstrate the applicability of the printer to produce medical image-based anatomic models (Figure 8). No complications were experienced during the printing process, although the manual removal of the support structures was challenging due to the adhesion between the silicone and the PLA.
Figure 8.
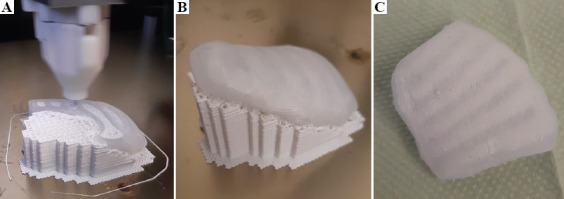
Silicone-PLA multi-material ribcage model based on a medical image of a newborn during printing (A), with support after printing (B), and after support removal (C).
4. Discussion
4.1. Overview of aims and results
The aim of this study was to build and test a 3D printer that enables features necessary for producing more realistic anatomic models in the future. The specifications stemming from this goal were printing multi-material structures out of at least one hard and one soft material, while also being capable of printing empty cavities, infill structures and thin-walled features. Considering the advantages and drawbacks of various AM methods and their applicable materials, a printer was built that combines FFF and DIW technologies to print with a single-component silicone rubber and a thermoplastic PLA filament.
The printing trials demonstrated that the established technology is capable of printing objects of both materials and can print silicone with a weakened internal structure (down-tuning) or combine silicone with PLA (up-tuning). An unsupported internal cavity and a thin-walled structure were also printable with the silicone. It was shown that the FFF printhead can create hard support structures. The strong adhesion between the PLA and the silicone that was experienced during the tests suggests that this material combination can be applied to create more complex multi-material structures, and that the silicone must be cut away from the PLA in case of using PLA for printing support structures under the silicone. These assumptions were confirmed through the last test, where the ribcage was indeed printable with PLA support and sufficient adhesion between the silicone and the PLA.
These promising outcomes imply that the technology could be used to approximate the mechanical properties of various soft biological tissues through up- and down-tuning strategies. Using the other half of the Viscotec printhead as a third extruder, the system could easily be extended to introduce a viscous fluid into printed internal cavities. Such a method used in parallel with up- and down-tuning strategies could possibly increase viscoelasticity[15] in anatomic models. This means that with the further development of our technology, a significant increase in anatomic model realism may be delivered, accelerating the development of certain medical instruments and improve medical education and preoperative planning.
4.2. Comparison with other technologies
From the perspective of down-tuning, the greatest limitation of concurrent technologies such as IJP, BJ, SLA, and DLP is their difficulty to realize completely closed and empty cavities, either because of an inherent need for support (IJP) or because of a leveled slurry or powder bath (BJ, SLA, DLP)[21]. This also applies to the Picsima silicone printing process[41], as well as the technology used by Wacker and ACEO[39], and by Spectroplast[43]. In our case, this limitation is overcome by utilizing extrusion-based DIW and FFF, even though the LAM process of Dow and GermanRepRap[40] is also free of this problem.
Considering up-tuning, IJP has a better resolution and a larger variety of applicable materials than our presented technology[14,16,17]. Our process can use up to three materials and can be extended to handle two more without changing the electronics. The LAM process of Dow and GermanRepRap[40] along with the one of Wacker and ACEO[39] could theoretically also be extended to work with multiple materials. Meanwhile, the other methods (BJ, SLA, DLP, Picsima, and Spectroplast) are more confined to single material printing[21,41,43].
The various available IJP printer models from Stratasys (including the J750 dedicated for anatomic models)[24] are frequently used in literature for producing soft multi-material tissue models[15-17]. However, the biological realism of the materials that are printable with these printers is often criticized, and IJP technologies are inherently limited in terms of printing unsupported overhangs. We have demonstrated that our system can print steeply overhanging structures, which (combined with other relevant features) may enable more accurate tissue approximation than what is possible with IJP systems.
Differentiating our system from other self-built silicone rubber printers, we can note that some are specialized on printing on curved surfaces[44,45], while others use two-component silicones on a heated building platform[46,47] and do not feature additional printheads for thermoplastics or other fluids.
4.3. Limitations and outlook
In case of DIW and FFF, spatial resolution, printing quality, and printing speed are tightly connected process parameters; therefore, the presented technology is slow compared to the shower-like droplet generation of IJP or BJ, or the scanning laser or full-layer projector of SLA or DLP. Moreover, despite the successful first prints, the system suffers from certain other limitations in its current state. If used in more complex geometries, the removal of PLA supports might damage the contact surface of the silicone. This adverse effect may be minimized by careful support design in the future. The difficulties with removing the silicone parts from the building platform may be eased by choosing a different printing surface. It must also be pointed out that the printing abilities were only demonstrated with one silicone and one thermoplastic material, and the general applicability to other materials is so far untested.
A decisive factor in the compatibility of a silicone-thermoplastic combination is the adhesion between them. Qualitatively testing the adhesion strength between the silicone and PLA or other thermoplastics remains an interesting direction for further research, along with the qualitative testing of the effects of infill structuring on the mechanical properties of silicone objects. Finally, the extent of applicability to the field of anatomic models depends not only on mechanical property tuning but also on geometric limitations. To succeed at printing complex anatomic geometries, an optimization method should first be developed to find the ideal printing parameters. This may be done in a follow-up study by printing various basic features at different printing settings and then analyzing the integrity and accuracy of the printed features. In addition, using the other available DIW extruder to deposit a high-viscosity filler liquid into internal cavities may provide a way to modify viscoelastic mechanical properties.
5. Conclusions
In this study, a novel multi-material AM technology targeted at facilitating the production of more realistic anatomical models was established and tested. The printable features enabled by this technology offer promising possibilities in the field of functional anatomic models. Analyzing geometric limitations, along with an evaluation of feasible mechanical properties, are needed before this technology could make a significant impact in the field of medical education, device testing, and pre-operative planning. However, a medical image-based anatomic model was already successfully printed in this study, implying a long-term applicability for the presented system. Moreover, besides anatomic models, the system may also have potential applications in the field of soft robotics, wearable electronic devices, or sports equipment.
Funding
This work was supported by the Provincial Government of Lower Austria (Land Niederösterreich) under grant assignment number WST3-F2-528983/005-2018.
Conflicts of interest
Concerning the manufacturing technology described in Materials and Methods subsections 2.1, 2.2 and 2.3, a patent application has been filed by the Austrian Center for Medical Innovation and Technology (ACMIT Gmbh) at the European Patent Office under applicant reference number 51241. The inventors are Laszlo Jaksa, Andrea Lorenz and Dieter Pahr.
Author contributions
L. J. designing and assembling the 3D-printer, conducting all tests, writing the manuscript D. P. consulting and supervising the design and publication processes G. K. consulting and supervising the design and publication processes A. L. consulting and supervising the design, assembly, testing and publication processes, managing component procurement and research budget.
References
- 1.Ventola CL. Medical Applications for 3D Printing:Current and Projected Uses. P T. 2014;39:704–11. [PMC free article] [PubMed] [Google Scholar]
- 2.Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D Printing Based on Imaging Data:Review of Medical Applications. Int J Comput Assist Radiol Surg. 2010;5:335–41. doi: 10.1007/s11548-010-0476-x. https://doi.org/10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 3.Wang K, Ho CC, Zhang C, et al. A Review on the 3D Printing of Functional Structures for Medical Phantoms and Regenerated Tissue and Organ Applications. Engineering. 2017;3:653–62. https://doi.org/10.1016/j.eng.2017.05.013. [Google Scholar]
- 4.Pietrabissa A, Marconi S, Negrello E, et al. An Overview on 3D Printing for Abdominal Surgery. Surg Endosc. 2019;34(1):1–13. doi: 10.1007/s00464-019-07155-5. https://doi.org/10.1007/s00464-019-07155-5. [DOI] [PubMed] [Google Scholar]
- 5.Preece D, Williams SB, Lam R, et al. Let's Get Physical:Advantages of a Physical Model Over 3D Computer Models and Textbooks in Learning Imaging Anatomy. Anat Sci Educ. 2013;6:216–24. doi: 10.1002/ase.1345. https://doi.org/10.1002/ase.1345. [DOI] [PubMed] [Google Scholar]
- 6.Khot Z, Quinlan K, Norman GR, et al. The Relative Effectiveness of Computer-based and Traditional Resources for Education in Anatomy. Anat Sci Educ. 2013;6:211–5. doi: 10.1002/ase.1355. [DOI] [PubMed] [Google Scholar]
- 7.Sulaiman A, Boussel L, Taconnet F, et al. In vitro Non-rigid Life-size Model of Aortic Arch Aneurysm for Endovascular Prosthesis Assessment. Eur J Cardiothorac Surg. 2008;33:53–7. doi: 10.1016/j.ejcts.2007.10.016. https://doi.org/10.1016/j.ejcts.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Giesel FL, Hart AR, Hahn HK, et al. 3D Reconstructions of the Cerebral Ventricles and Volume Quantification in Children with Brain Malformations. Acad Radiol. 2009;16:610–7. doi: 10.1016/j.acra.2008.11.010. https://doi.org/10.1016/j.acra.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Golab A, Smektala T, Kaczmarek K, et al. Laparoscopic Partial Nephrectomy Supported by Training Involving Personalized Silicone Replica Poured in Three-Dimensional Printed Casting Mold. J Laparoendosc Adv Surg Tech A. 2017;27:420–2. doi: 10.1089/lap.2016.0596. https://doi.org/10.1089/lap.2016.0596. [DOI] [PubMed] [Google Scholar]
- 10.Mavili ME, Canter HI, Sağlam-Aydinatay B, et al. Use of Three-Dimensional Medical Modeling Methods for Precise Planning of Orthognathic Surgery. J Craniofac Surg. 2007;18:740–7. doi: 10.1097/scs.0b013e318069014f. https://doi.org/10.1097/scs.0b013e318069014f. [DOI] [PubMed] [Google Scholar]
- 11.Poukens J, Haex J, Riediger D. The Use of Rapid Prototyping in the Preoperative Planning of Distraction Osteogenesis of the Cranio-maxillofacial Skeleton. Comput Aided Surg. 2003;8:146–54. doi: 10.3109/10929080309146049. https://doi.org/10.3109/10929080309146049. [DOI] [PubMed] [Google Scholar]
- 12.Tack P, Victor J, Gemmel P, et al. 3D-Printing Techniques in a Medical Setting:A Systematic Literature Review. Biomed Eng Online. 2016;15:115. doi: 10.1186/s12938-016-0236-4. https://doi.org/10.1186/s12938-016-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Q, Dong H, Su J, et al. A Review of 3D Printing Technology for Medical Applications. Engineering. 2018;4:729–42. [Google Scholar]
- 14.Qian Z, Wang K, Liu S, et al. Quantitative Prediction of Paravalvular Leak in Transcatheter Aortic Valve Replacement Based on Tissue-Mimicking 3D Printing. JACC Cardiovasc Imaging. 2017;10:719–31. doi: 10.1016/j.jcmg.2017.04.005. https://doi.org/10.1016/j.jcmg.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Ratinam R, Quayle M, Crock J, et al. Challenges in Creating Dissectible Anatomical 3D Prints for Surgical Teaching. J Anat. 2019;234:419–37. doi: 10.1111/joa.12934. https://doi.org/10.1111/joa.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K, Wu C, Qian Z, et al. Dual-material 3D Printed Metamaterials with Tunable Mechanical Properties for Patient-specific Tissue-mimicking Phantoms. Addit Manuf. 2016;12:31–7. https://doi.org/10.1016/j.addma.2016.06.006. [Google Scholar]
- 17.Wang K, Zhao Y, Chang YH, et al. Controlling the Mechanical Behavior of Dual-material 3D Printed Meta-materials for Patient-specific Tissue-mimicking Phantoms. Mater Des. 2016;90:704–12. https://doi.org/10.1016/j.matdes.2015.11.022. [Google Scholar]
- 18.Goh GL, Zhang H, Chong TH, et al. 3D Printing of Multilayered and Multimaterial Electronics:A Review. Adv Electron Mater. 2021;2021:2100445. https://doi.org/10.1002/aelm.202100445. [Google Scholar]
- 19.Yap YL, Sing SL, Yeong WY. A Review of 3D Printing Processes and Materials for Soft Robotics. Rapid Prototyp J. 2020;26:1345–61. https://doi.org/10.1108/rpj-11-2019-0302. [Google Scholar]
- 20.Qiu K, Haghiashtiani G, McAlpine MC. 3D Printed Organ Models for Surgical Applications. Annu Rev Anal Chem. 2018;11:287–306. doi: 10.1146/annurev-anchem-061417-125935. https://doi.org/10.1146/annurev-anchem-061417-125935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngo TD, Kashani A, Imbalzano G, et al. Additive Manufacturing (3D Printing):A Review of Materials, Methods, Applications and Challenges. Compos B Eng. 2018;143:172–96. https://doi.org/10.1016/j.compositesb.2018.02.012. [Google Scholar]
- 22.Pugliese L, Marconi S, Negrello E, et al. The Clinical Use of 3D Printing in Surgery. Updates Surg. 2018;70:381–8. doi: 10.1007/s13304-018-0586-5. https://doi.org/10.1007/s13304-018-0586-5. [DOI] [PubMed] [Google Scholar]
- 23.Dorweiler B, Baqué PE, Chaban R, et al. Quality Control in 3D Printing:Accuracy Analysis of 3D-Printed Models of Patient-Specific Anatomy. Materials. 2021;14:1021. doi: 10.3390/ma14041021. https://doi.org/10.3390/ma14041021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stratasys Ltd. [Last accessed on 2021 Jan 10]. Available from: https://stratasys.com/medical/advanced-medical-models .
- 25.Mirzaali MJ, Nava AH, Gunashekar D, et al. Mechanics of Bioinspired Functionally Graded Soft-hard Composites Made by Multi-material 3D Printing. Composit Struct. 2020;237:111867. doi: 10.3390/ma12172735. https://doi.org/10.1016/j.compstruct.2020.111867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ionita CN, Mokin M, Varble N, et al. Challenges and Limitations of Patient-specific Vascular Phantom Fabrication Using 3D Polyjet Printing. Proc SPIE Int Soc Opt Eng. 2014;9038:90380M. doi: 10.1117/12.2042266. https://doi.org/10.1117/12.2042266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiter M, Major Z. A Combined Experimental and Simulation Approach for Modelling the Mechanical Behaviour of Heterogeneous Materials Using Rapid Prototyped Microcells. Virtual Phys Prototyp. 2011;6:111–20. https://doi.org/10.1080/17452759.2011.586949. [Google Scholar]
- 28.Hiller J, Lipson H. Tunable Digital Material Properties for 3D Voxel Printers. Rapid Prototyp J. 2010;16:241–7. https://doi.org/10.1108/13552541011049252. [Google Scholar]
- 29.Patent. Additive Manufacturing of Rubber-like Materials. Patent US2019224914A1 2019 [Google Scholar]
- 30.Slesarenko VY. Towards Mechanical Characterization of Soft Digital Materials for Multimaterial 3D-Printing. Int J Eng Sci. 2017;123:62–72. https://doi.org/10.1016/j.ijengsci.2017.11.011. [Google Scholar]
- 31.Goh GL, Agarwala S, Yong WI. 3D Printing of Microfludic Sensor for Soft Robots:A Preliminary Study in Design and Fabrication. Proceedings of the 2nd International Conference on Progress in Additive Manufacturing (Pro-AM 2016) 2016:177–81. [Google Scholar]
- 32.Truby RL, Lewis JA. Printing Soft Matter in Three Dimensions. Nature. 2016;540:371–8. doi: 10.1038/nature21003. https://doi.org/10.1038/nature21003. [DOI] [PubMed] [Google Scholar]
- 33.Yeo J, Koh J, Wang F, et al. Silicon Containing Hybrid Copolymers. Vol. 2020. Germany: Wiley-VCH Verlag GmbH &Co. KGaA; 2020. 3D Printing Silicone Materials and Devices; pp. 239–63. https://doi.org/10.1002/9783527823499.ch9. [Google Scholar]
- 34.Zhao Y, Yao R, Ouyang L, et al. Three-dimensional Printing of Hela Cells for Cervical Tumor Model In Vitro. Biofabrication. 2014;6:035001. doi: 10.1088/1758-5082/6/3/035001. https://doi.org/10.1088/1758-5082/6/3/035001. [DOI] [PubMed] [Google Scholar]
- 35.Lee JM, Sing SL, Yeong WY. Bioprinting of Multimaterials with Computer-aided Design/Computer-aided Manufacturing. Int J Bioprint. 2020;6:245. doi: 10.18063/ijb.v6i1.245. https://doi.org/10.18063/ijb.v6i1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W, Zhang YS, Heinrich MA, et al. Rapid Continuous Multimaterial Extrusion Bioprinting. Adv Mater. 2016;29:1604630. doi: 10.1002/adma.201604630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardin JO, Ober TJ, Valentine AD, et al. Microfluidic Printheads for Multimaterial 3D Printing of Viscoelastic Inks. Adv Mater. 2015;27:3279–84. doi: 10.1002/adma.201500222. https://doi.org/10.1002/adma.201570145. [DOI] [PubMed] [Google Scholar]
- 38.Skylar-Scott MA, Mueller J, Visser CW, et al. Voxelated Soft Material Via Multimaterial Multinozzle 3D Printing. Nature. 2019;575:330–4. doi: 10.1038/s41586-019-1736-8. https://doi.org/10.1038/s41586-019-1736-8. [DOI] [PubMed] [Google Scholar]
- 39.Wacker Chemie AG. Avaialble from: https://www.aceo3d.com/3d-printing . 2021. [Last accessed on 2021 Jan 10].
- 40.GermanRepRap GmbH. 2021. [Last accessed on 2021 Jan 10]. Avaialble from: https://www.germanreprap.com/material-de/SILASTIC-3D-3335.aspx .
- 41.Fripp Design Ltd. 2021. [Last accessed on 2021 Jan 10]. Avaialble from: https://www.picsima.com .
- 42.Deltatower GmbH. 2021. [Last accessed on 2021 Jan 10]. Avaialble from: https://www.deltatower.ch/en/home-2 .
- 43.Spectroplast AG. 2021. [Last accessed on 2021 Jan 10]. Avaialble from: https://www.spectroplast.com .
- 44.Coulter F. 2021. [Last accessed on 2021 Jan 10]. Avaialble from: http://www.fergalcoulter.eu .
- 45.Coulter F, Schaffner M, Faber J, et al. Bioinspired Heart Valve Prosthesis Made by Silicone Additive Manufacturing. Matter. 2019;1:266–79. https://doi.org/10.1016/j.matt.2019.05.013. [Google Scholar]
- 46.Luis E, Pan HM, Sing SL, et al. Silicone 3D Printing:Process Optimization, Product Biocompatibility, and Reliability of Silicone Meniscus Implants. 3D Print Addit Manufact. 2019;6:319–32. https://doi.org/10.1089/3dp.2018.0226. [Google Scholar]
- 47.Luis E, Pan HM, Sing SL, et al. 3D Direct Printing of Silicone Meniscus Implant Using a Novel Heat-Cured Extrusion-Based Printer. Polymers. 2020;12:1031. doi: 10.3390/polym12051031. https://doi.org/10.3390/polym12051031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patent. 3D-printing Device and Process for Producting an Object with Use of a 3D-Printing Device, Patent WO2017108208A1 2017 [Google Scholar]
- 49.Patent. 3D Printing Method Utilizing Heat-curable Silicone Composition, Patent WO2017040874A1 2017 [Google Scholar]
- 50.Studart AR. Additive Manufacturing of Biologically-inspired Materials. Chem Soc Rev. 2016;45:359–76. doi: 10.1039/c5cs00836k. https://doi.org/10.1039/c5cs00836k. [DOI] [PubMed] [Google Scholar]
- 51.Bakarich SE, Gorkin R, Panhuis M, et al. Three-Dimensional Printing Fiber Reinforced Hydrogel Composites. ACS Appl Mater Interfaces. 2014;6:15998–16006. doi: 10.1021/am503878d. https://doi.org/10.1021/am503878d. [DOI] [PubMed] [Google Scholar]
- 52.Viscotec GmbH. 2021. [Last accessed on 2021 Jan 10]. Avaialble from: https://www.viscotec.de/produkte/3d-druckkoepfe .
- 53.White JS, Akens T. 2021. [Last accessed on 2021 Jan 10]. Avaialble from: https://railcore.org .
- 54.Duet3D Advanced 3D Printing Electronics. 2021. [Last accessed on 2021 Jan 10]. Avaialble from: https://www.duet3d.com/DuetWifi .
- 55.Prusa Research. 2021. [Last accessed on 2021 Jan 10]. Avaialble from: https://www.prusa3d.com/prusaslicer .


