Abstract
Background
Novel cancer immunotherapy seeks to harness the body's own immune system and tip the balance in favour of antitumour activity. The intracellular enzyme indoleamine 2,3‐dioxygenase (IDO) is a critical regulator of the tumour microenvironment (TME) via tryptophan metabolism. The potential immunotherapeutic role of IDO in head and neck squamous cell carcinoma (HNSCC) requires further exploration. We aim to assess the evidence on IDO in HNSCC.
Methods
A systematic review of literature and clinical trials databases.
Results
We included 40 studies: seven involved cell lines: eight assessed tumour immunohistochemistry: ten measured IDO gene transcription: 15 reported on clinical trials. Increased cell line IDO expression was postulated to adversely affect tumour metabolism and apoptosis. Immunohistochemical IDO expression correlated with worse survival. Gene transcription studies associated IDO with positive PD‐L1 and human papillomavirus (HPV) status. Phase I/II clinical trials showed (a) overall response (34%‐55%) and disease control rates (62%‐70%) for IDO1 inhibitor in combination with a PD‐1 inhibitor, (b) similar safety profiles when both are used in combination therapy compared to each as monotherapies and (c) IDO gene expression as a predictive biomarker for response to PD‐L1 therapy.
Conclusions
IDO expression is increased in the TME of HNSCC, which correlates with poor prognosis. However, the exact mechanism of IDO‐driven immune modulation in the TME is an enigma. Future translational studies should map IDO activity during HNSCC treatment and elucidate its precise role in the TME, such research will underpin the development of clinical trials establishing the efficacy of IDO inhibitors in HNSCC.
Keywords: biomarkers; immune system; immunotherapy; indoleamine‐pyrrole 2,3‐dioxygenase; squamous cell carcinoma of head and neck; tryptophan; tumour microenvironment
Keypoints.
IDO is integral to TME immunity in HNSCC particularly in HPV‐positive cancers.
IDO can be used to modulate existing therapies and has applications in combinatorial immunotherapy.
Retrospective studies have shown its presence in the TME and suggest a link to HNSCC treatment outcome.
However, the exact mechanism of IDO‐driven immune modulation in the HNSCC TME remains unclear.
We now require prospective longitudinal studies to track IDO activity and expression throughout HNSCC treatment, thence optimise IDO‐based immunotherapy.
1. INTRODUCTION
The burgeoning field of cancer immunotherapy has made significant progress with the application of immune checkpoint inhibitors in the treatment of solid tumours at different sites across the body. Such therapeutic strategies seek to harness the body's own immune system and tip the balance in favour of antitumour immunity. The first clinically validated immune checkpoint therapy targeting cytotoxic T‐lymphocyte‐associated antigen (CTLA‐4) mediated tumour regression and increased overall survival in melanoma patients, but was associated with frequent immune‐related adverse events. 1 , 2 , 3 , 4 , 5 Programmed death 1 (PD‐1) protein and its ligand PD‐L1 was subsequently discovered 6 , 7 and shown to have good safety and efficacy in inducing durable tumour regression and prolonged stable disease in patients with advanced cancers including non‐small cell lung cancer (NSCLC), melanoma, renal cell, ovarian, colorectal, pancreatic, gastric and breast cancer. 8 However, the vast majority of head and neck cancer patients, about 80%, remain unresponsive to immune checkpoint inhibitor therapy, highlighting the need for more effective immunotherapies and predictive biomarkers. 9 IDO1 inhibitors for melanoma, glioblastoma, NSCLC, pancreatic and breast cancer are under investigation by pharmaceutical companies and sponsors. 10 To date, IDO inhibitors for head and neck cancer have been tested in only several published clinical studies. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18
Head and neck squamous cell carcinoma (HNSCC) is the sixth leading cause of cancer worldwide and is diagnosed in 8000 new patients annually in the UK. 19 HNSCCs are divided into two clinically, genomically and immunologically distinct subgroups based on their association with human papillomavirus (HPV) infection: (a) the majority of HNSCCs are HPV‐negative and tend to present in older patients, usually with a history of smoking and alcohol use; their tumours are often characterised by p53 mutations and have poor 5‐year survival ranging from 33.8% to 66.8% depending on subsite, whilst (b) HPV‐positive HNSCCs arise mainly in younger, Caucasian, non‐smokers and their tumours are characterised by integration of viral genome and the expression of E6 and E7 viral oncoproteins which result in the inactivation of p53 and retinoblastoma (Rb) protein, and subsequent overexpression of p16, but better prognosis and overall survival as their tumours are often radiosensitive. 20 Current surgical and non‐surgical treatments for HNSCC have devastating functional and cosmetic consequences. Survival has improved little in the past four decades, that is most less than 50%. 21 The head and neck tumour microenvironment (TME) is a site of intense immunological activity, driving a recent emergence in immunotherapy being applied to HNSCC.
Indoleamine 2,3‐dioxygenase (IDO) is an intracellular enzyme which plays a critical role in the immunity of the TME via tryptophan metabolism. Its activity is increased in the TME of many cancers and its expression was found to be a negative prognostic indicator in melanoma, 22 ovarian, 23 colorectal 24 and lung cancer. 25 , 26 IDO inhibits natural and therapy‐induced antitumour immunity as it catabolises the amino acid tryptophan to generate kynurenine and other immunosuppressive catabolites which activate Foxp3 regulatory T cells and attenuate effector T‐cell responses to inhibit immune‐mediated killing of tumour cells. Due to its important role in TME immunity, IDO is an immune checkpoint which can be potentially exploited to improve treatment outcomes. However, the immunotherapeutic role of IDO in HNSCC requires further exploration.
Our review aims to systematically assess the current literature for pre‐clinical and clinical evidence on the immunotherapeutic role of IDO in HNSCC. Our objectives were to (a) identify all studies which investigated IDO in HNSCC, (b) identify studies which investigated IDO activity and/or expression in HNSCC, (c) evaluate the effectiveness of IDO inhibitors at improving the outcomes of patients with HNSCC, (d) compare the use of IDO inhibitors alone and in combination with other treatments for HNSCC and (e) evaluate the potential for immunotherapeutic strategies involving the IDO pathway in the treatment of HNSCC.
2. MATERIALS AND METHODS
2.1. Data sources and literature search
A systematic literature search was conducted in Ovid MEDLINE, Ovid Embase, Scopus, Web of Science, Cochrane library and ClinicalTrials.gov databases from inception until present day. The PRISMA guidelines for study selection were followed. 27 All studies that evaluated the involvement of IDO in HNSCC were systematically retrieved. The following search terms and strategy was used: (“indoleamine 2,3‐dioxygenase” OR “IDO” OR “IDO1” OR “IDO‐1” OR “IDO2” OR “IDO‐2”) AND (“squamous cell carcinoma” OR “squamous cell cancer” OR “SCC”). The titles and abstracts from the initial search results were screened independently by two authors DJL and JCKN. To ensure inclusion of all studies related to HNSCC, DJL and JCKN manually screened the studies with squamous cell carcinoma to include those involving the head and neck region.
2.2. Study selection
In the initial screening, the following criteria were required for inclusion: (a) HNSCC from any head and neck subsite (oral, oropharynx, nasopharynx, larynx and hypopharynx), (b) study of IDO expression or activity, (c) all study types (prospective or retrospective, observational or experimental, pre‐clinical or clinical), (d) published in English language only and (e) original articles and conference abstracts. Duplicates, correspondence, review articles and studies without data on IDO in the context of HNSCC were excluded.
2.3. Data extraction and analysis
Following the generation of a list of articles meeting the inclusion criteria, DJL and JCKN each performed an in‐depth review of the studies and extracted data for comparison. Similar studies were grouped together for qualitative analysis.
3. RESULTS
3.1. Included studies
A total of 273 studies were identified from databases and seven studies from additional sources, and 146 were screened after removal of 134 duplicates. A total of 100 studies were excluded with reasons described in the PRISMA flow diagram in Figure 1. After full‐text review, 40 studies were included in the final analysis.
FIGURE 1.
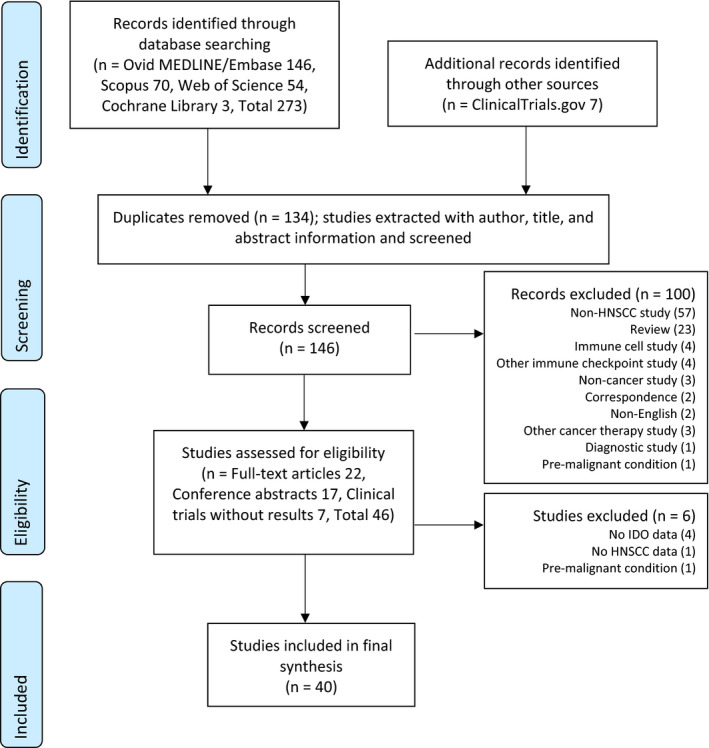
PRISMA flow diagram for study selection. Abbreviations: IDO, indoleamine 2,3‐dioxygenase; HNSCC, head and neck squamous cell carcinoma
Evidence from pre‐clinical and clinical studies involving IDO was extracted. A total of 22 full‐text articles, 17 conference abstracts and seven clinical trials without results were evaluated. Of those seven studies involved cell lines, eight assessed tumour immunohistochemistry (IHC), 10 were IDO gene transcription studies, and 15 others reported on clinical trials (eight published, seven registered without results). All seven cell line studies used different HNSCC cell lines. The prognostic studies involving IHC investigated IDO expression in HNSCC at different subsites, defined as; lower lip, oral cavity, tongue and larynx. The clinical trials compared survival with combination therapies involving IDO1 inhibitor vs monotherapy using PD‐1 inhibitor Pembrolizumab, and assessed IDO expression value as a predictive biomarker for response to PD‐L1 therapy. Additionally, 13 conference abstracts were identified and summarised in Table S1.
3.2. Cell line studies on IDO in HNSCC
The seven studies which investigated IDO in HNSCC cell lines, each is an immortalised cell culture developed from a single human HNSCC tumour, are summarised in Table 1. Of those, the majority investigated IDO in oral and oropharyngeal HNSCC cell lines. The studies were heterogeneous in the method of cell line analysis, which ranged from enzyme‐linked immunosorbent assay (ELISA), 2D/3D cell culture, computational simulation model, enzymatic IDO activity assay, quantitative reverse transcriptase polymerase chain reaction (qRT‐PCR), and IFNγ stimulation followed by treatment with cytostatic drugs and quantification of metabolites generated via IDO activity by liquid chromatography tandem mass spectrometry. Additionally, the same cell lines SCC4, SCC15 and SCC25 were only used in 3 of the 7 studies. Bates 28 showed that SCC15 (T4N1M0) produced significantly more IDO than any other cell line (SCC4, SCC25, UM‐SCC19, UM‐SCC 84, UM‐SCC 92 and UM‐SCC 99). Interestingly in that group, SCC15 was the only cell line derived from T4 stage HNSCC. However, in the computational simulation model 29 SCC4 had significantly higher IDO expression compared to SCC15 and SCC25. The SCC4 cell line was classified as a non‐responder compared to SCC15 and SCC25 which were classified as responders to PD‐L1 immunotherapy. This characteristic of SCC4 was supported by observations from Liang et al 30 showing that stimulator of interferon genes (STING) activation significantly induced IDO expression in SCC4. It is also postulated that IDO activity may interfere with tumour metabolism. The studies by Subramanian 31 and El Jamal 32 also support the central role of IDO inhibition in upregulation of genes in apoptosis and activation of apoptotic pathways through the suppression of haem oxygenase‐1 and accumulation of reactive oxygen species. Furthermore, Riess et al 33 used the experimental cyclin‐dependent kinase inhibitor Dinaciclib to suppress IDO activity, thus reducing tryptophan metabolism via the kynurenine pathway in HNSCC cell lines, whilst chemotherapeutics tend to activate this pathway. Chemotherapy may modulate IDO activity indirectly by inducing stressed and dying cells to release damage‐associated molecular patterns (DAMPs), which are sensed to stimulate inflammatory responses. Nevertheless, these findings emphasise the limitations of conventional therapies and the potential of targeted therapies to interfere with HNSCC metabolism. Al‐Samadi et al 34 showed that applying an IDO1 inhibitor in an in vitro 3D microfluidic chip assay with HSC‐3 induced immune cell migration towards cancer cells. Thus, changing the tumour microenvironment from immunologically “cold” (ie inactive) to “hot” (ie active/inflamed) could enhance the efficacy of other immunotherapeutic drugs in combination.
TABLE 1.
Cell line studies on IDO in HNSCC
| First author, year (country) | Journal | Cell line (subsite) | TNM stage | Assay method | Results interpretation |
|---|---|---|---|---|---|
| Riess, 33 2020 (Germany) | Frontiers in Immunology |
Hypopharyngeal: FADU Pharyngeal: Detroit‐562 Tongue: Cal‐33 PE/CA/PJ‐15 UT‐SCC‐14 UT‐SCC‐15 |
— | HNSCC cell lines were cultured and treated with IFNγ for 24 h and 72 h, then treated with cytostatic drugs including 5‐fluorouracil (5‐FU), Cisplatin, Gemcitabine and Cetuximab. IDO1 immunofluorescence was performed on the treated cells and kynurenine pathway (KP) metabolites in the cell culture supernatant was quantified by liquid chromatography tandem mass spectrometry | IDO1 expression was low, but inducible upon IFNγ treatment of HNSCC cells. Upon treatment with 5‐FU, Gemcitabine and Cetuximab, IDO1 and additional genes of the KP (KYAT1, KYAT2 and KMO) were induced. Cyclin‐dependent kinase inhibitor Dinaciclib suppressed the KP, whilst conventional chemotherapeutics tend to activate the KP |
| Al‐Samadi, 34 2019 (Finland) | Experimental Cell Research |
Oral cavity: tongue: HSC‐3 |
— | In vitro 3D microfluidic chip assay. HSC‐3 was embedded in human tumour‐derived matrix along with patients’ serum, cancer and immune cells, which were then loaded with anti‐PD‐L1 and IDO1 inhibitors. Immune cell migration and cancer cell proliferation rates were evaluated | IDO1 inhibitor induced immune cell migration towards cancer cells in HSC‐3 and two HNSCC patient samples, which could change the tumour from “cold” to “hot” and enhance the efficacy of other immunotherapeutic drugs in combination. This in vitro 3D microfluidic chip assay could be used to further test immunotherapeutic drugs against patient samples |
| Bates, 28 2018 (USA) | Translational Cancer Research |
Oral cavity: tongue: SCC4 SCC15 SCC25 Oropharynx: base of tongue: UM‐SCC19 Oral cavity: UM‐SCC84 Oral cavity: lateral tongue: UM‐SCC92 Oropharynx: UM‐SCC99 |
T3N0M0 T4N1M0 T2N1 T2N1M0 T2N0M0 T2N0M0 T3N0M0 |
ELISA to determine the concentration of IDO in cell lysates | SCC15 produced significantly more IDO than any of the six other cell lines. HNSCC cell lines from different hosts can have varying amounts of biomarkers. These differences could be due to the stage of disease, site of tumour, tissue type or genomic differences between patients. These results support personalised medicine in treating HNSCC |
| Subramanian, 31 2018 (USA) | Cancer Research | Cell line not specified | — | HNSCC cell lines grown in 2D culture. Kynurenine levels measured by MS. IDO1 levels in tissue measured by Western Blot | High levels of kynurenine in HNSCC cell lines shown through metabolic profiling via MS. Checkpoint inhibition of IDO1 leads to an upregulation of genes in glycolysis (ACLY, G6PD, COX5A, LPL and PFKL) and apoptosis (CASP7, CASP9, BCL2L11) in vitro |
| Bates, 29 2017 (USA) | Oral Surgery Oral Medicine Oral Pathology Oral Radiology |
Oral cavity: tongue: SCC4 SCC15 SCC25 |
T3N0M0 T4N1M0 T2N1 |
Cell line‐specific predictive computational simulation models used to predict expression of IDO1 | Predicted IDO expression in SCC4 (17.29%), SCC15 (2.75%) and SCC25 (4.97%) with respect to controls. In the simulation model, SSC4 was classified as a non‐responder whilst SCC15 and SCC25 were classed as responders to PD‐L1 immunotherapy |
| El Jamal, 32 2016 (USA) | Cell Division |
HNSCC Mouth: CLS‐354 SCC nasal septum: RPMI 2650 |
— | Enzymatic IDO activity assay, absorbance at 490 nm. Immunoblot with anti‐IDO antibody 1:500 (BioGenes, Berlin, Germany), Western Blot | Described central role of IDO in IFNγ‐induced apoptosis of HNSCC cells by the suppression of HO‐1 leading to the accumulation of ROS and activation of apoptotic pathways |
| Liang, 30 2015 (China) | Biochimica et Biophysica Acta ‐ Molecular Basis of Disease |
Oral SCC: HSC‐3 SCC‐4 Normal human keratinocyte: HaCaT |
— | IDO mRNA isolation by qRT‐PCR | IDO expression was significantly induced in HaCaT, HSC‐3 and SCC4 by STING activation. Suggests the establishment of HNSCC TME by immunosuppressive cytokines such as IDO could be promoted by 2′‐3′ cGAMP activation of STING |
Abbreviations: ELISA, enzyme‐linked immunosorbent assay; TNM, Tumour Node Metastasis; SCC, squamous cell carcinoma; MS, mass spectrometry; HO‐1, haem oxygenase‐1; ROS, reactive oxygen species; STING, stimulator of interferon genes; qRT‐PCR, quantitative reverse transcription polymerase chain reaction; cGAMP, 2′‐5′,3′‐5′cyclic AMP‐GMP.
3.3. Tumour immunohistochemistry studies on IDO in HNSCC
The majority of tumour IHC studies were performed on formalin‐fixed paraffin‐embedded tissue blocks. The studies in this group were designed with a retrospective method of analysis; none used prospective, fresh tissue collection or analysis of IDO activity within the tissues. The tissues studied came from the lower lip, oral cavity, tongue, tonsil and larynx. The eight studies included in this group are summarised in Table 2. Anti‐IDO monoclonal antibody clone 10.1 was widely used. IDO staining was most commonly seen at the invasive front of the tumour and increased IDO staining correlated with worse survival. 35 , 36 , 37 Four of the studies 35 , 36 , 37 , 38 were prognostic studies which reported on IDO expression and correlation with outcome. Only two studies fulfilled all REMARK checklist criteria for prognostic biomarker studies. In a recent prognostic study by Wang et al, an increase in IDO expression was seen in clinical non‐responders to Nimotuzumab (anti‐epidermal growth factor receptor) therapy, suggesting that IDO may be a biomarker of immune status in the TME during therapy in oral SCC patients. 38
TABLE 2.
Tumour immunohistochemistry studies on IDO in HNSCC
| First author, year (country) | Tumour site/stage | Source/preparation | Primary antibody, manufacturer (clone), dilution | Cases (n) | Method of analysis | Results interpretation | Compliance with REMARK 77 checklist |
|---|---|---|---|---|---|---|---|
| Wang, 38 2019 (China) | Oral SCC (OSCC) | FFPE tissue sections collected before and after 4‐wk treatment with six cycles of Nimotuzumab | Anti‐IDO1 (Abcam, ab211017), 1:1500 | 36 | Retrospective. IHC slides were assessed by 3 independent pathologists blindly and a staining score was given | Nimotuzumab therapy increased the expression of IDO in the TME of OSCC patients compared with baseline pre‐treatment P = .0053, suggesting IDO as a marker of immune status | Checklist items 6 and 9 not fulfilled |
| Succaria, 78 2018 (USA) | HNSCC, unspecified | Archived HNSCC specimen | Unspecified | 27 | Retrospective. IDO expression by tumour cells and infiltrating immune cells | >500 IDO+expressing cells/mm2 in 17/27 HNSCC specimens, IDO expressed by tumour cells and infiltrating immune cells in 12/27 (44%) cases (range 5%‐95% tumour cells+) | — |
| Venkata, 79 2017 (India) | HNSCC, unspecified | FFPE | — | 50 | Retrospective. Stained slides analysed manually and by digital algorithms | Increased expression of IDO in tumour cells correlated with FOXP3‐positive immune cells. Overall percentage of IDO and CD8‐positive immune cells were higher than PD‐L1 and FOXP3‐positive immune cells | — |
| Seppälä, 35 2016 (Finland) | Tongue SCC (58) and lymph node samples (32), control group (30) with tongue squamous cell hyperplasia | FFPE | Anti‐IDO monoclonal antibody, (MAB5412) 10.1, 1:200 | 108 | Retrospective. Semi‐quantitative light microscopic evaluation by two observers. IDO proportion and IDO staining intensity scores were calculated | IDO expression was higher in tongue hyperplasia than SCC. In tumour stage T2‐T4 and tumours with strong inflammation at the invasive front, IDO expression correlated with poor survival | Fulfilled all items |
| Ye, 36 2013 (China) | Laryngeal SCC: Glottic (92), Subglottic (65); Stage: Early I‐II (78), Late III‐IV (109) | FFPE surgical specimen, tissue block with tumour cells and non‐neoplastic laryngeal tissue was selected | IDO, Chemicon (AB5968) 10.1, 1:300 | 187 | Retrospective. IDO staining intensity in the tissue and tumour‐infiltrating lymphocytes. Correlation with survival analysis | Tumour IDO expression not significantly correlated with histology, clinical/nodal stage or tumour differentiation, but positively associated with density of FOXP3+ TILs (P = .028). High IDO expression was an independent predictor of poor DFS (HR = 3.973, P = .026) and OS (HR = 3.258, P = .029) | Fulfilled all items |
| Kuales, 80 2011 (Germany) | Lower lip SCC | Lesional biopsies, FFPE | Anti‐IDO monoclonal antibody, Millipore (10.1), 1:150 | 47 | Retrospective. Density of inflammatory infiltrate at the invasive front of each tumour was calculated | IDO expression correlated with moderate to intense inflammatory infiltrate and was found in myeloid CD11c+ S100+ DCs along the border of invasive tumour cells where Foxp3 regulatory T cells were also present | — |
| Laimer, 37 2011 (Austria) | Oral SCC | Paraffin blocks, deparaffinised and rehydrated sections mounted on slides | Anti‐IDO human antibody, Chemicon, Millipore (ab9252) sheep polyclonal, 1:500 | 88 | Retrospective. IDO expression was evaluated and total expression score given. Cox proportional hazard model for the relationship of IDO expression with survival time | IDO expression, staging, tumour grade 3 were prognostic for poorer overall survival. IDO was a prognostic factor in patients who received adjuvant (radio)chemotherapy, but had no impact in patients without adjuvant therapy | Checklist items 6 and 9 not fulfilled |
| Ferdinande, 81 2008 (Belgium) | Tonsil SCC (26), Tongue SCC (12) | Unspecified | — | 38 | Inflammatory infiltrate evaluated and scored (semi)quantitatively | 73% of tonsil SCC and 92% of tongue SCC showed IDO expression in tumour cells, focally at invasive front, and no association was found with TNM stage. IDO was present mostly in DCs | — |
Abbreviations: REMARK, Reporting Recommendations for Tumour Marker Prognostic Studies 77 ; SCC, squamous cell carcinoma; FFPE, formalin‐fixed paraffin‐embedded; IHC, immunohistochemistry; TIL, tumour‐infiltrating lymphocytes; DFS, disease‐free survival; DC, dendritic cell.
3.4. IDO gene transcription studies in HNSCC
We included 10 studies which investigated IDO gene transcription, summarised in Table 3. A variety of sources including tissue and blood specimen, 3D tumour microspheres, The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) were used to acquire transcription data for analysis. Methods of analysis included single‐sample gene set enrichment analysis (ssGSEA), NanoString analysis, MassARRAY, quantitative polymerase chain reaction (qPCR) and gene expression analysis in peripheral blood mononuclear cells (PBMCs). IDO was strongly expressed in human papillomavirus (HPV) positive HNSCCs and correlated with E7 HPV antigen expression. 39 IDO1 was overexpressed in tumours from never‐smokers and never‐drinkers; and gene expression profiles showed that IDO1 together with PD‐L1 were co‐overexpressed in HNSCCs 40 , 41 , 42 compared to IDO1 presence in normal head and neck tissue. 42 Recent studies of methylation of CpG sites suggest that IDO1 expression levels are epigenetically regulated by DNA methylation and hypermethylation of IDO1 is associated with poor overall survival. 43 , 44 Measuring expression during treatment, a significant (3.6‐fold) increase was seen in IDO expressed in PBMCs during radiotherapy for patients with stage III‐IV HNSCC. 45 Whereas after chemoradiation treatment, IDO1 mRNA levels correlated with worse overall survival, and a combined decrease in expression of PD‐L1 and IDO1 post‐treatment was associated with better progression‐free and overall survival. 46
TABLE 3.
IDO gene transcription studies in HNSCC
| First author, year (country) | Cases (n), source | Method of analysis | Results interpretation |
|---|---|---|---|
| Economopoulou, 46 2020 (Greece) | 113 locally advanced HNSCC patients who underwent cisplatin chemoradiation, peripheral blood collected at baseline and 1 wk after end of treatment | Expression of IDO1 in the EpCAM+CTC fraction before and after cisplatin chemoradiation. Multivariate Cox regression analysis was used to assess the prognostic value of PD‐L1 and IDO1 expression | IDO1 was significantly overexpressed at baseline compared to post‐treatment (P = .007). IDO1 mRNA expression at baseline was associated with better PFS (HR = 0.19, P = .017). Post‐treatment IDO1 mRNA levels correlated with worse OS (HR = 3.27, P = .008). Patients with combined decreased expression of PD‐L1 and IDO1 post‐treatment had better PFS (P = .043) and OS (P = .021) |
| Sailer, 44 2019 (Germany) | 528 HNSCC patients, TCGA; and 138 HNSCC patients as a validation cohort from the University Hospital Bonn | Methylation of 3 CpG sites was correlated with mRNA expression, immune cell infiltration, mutational burden, HPV status and OS | IDO1 methylation and IDO1 mRNA expression were inversely correlated in the promotor and promoter flank region. IDO1 promoter flank hypermethylation was associated with poor OS (P < .001). These suggest that IDO1 expression levels are epigenetically regulated by DNA methylation |
| Lecerf, 42 2019 (France) | 96 HNSCC patients who underwent primary surgery | Real‐time polymerase chain reaction was used to assess the expression of 46 immune‐related genes | IDO1 (75%) was among the most significantly overexpressed immune‐related genes and had significantly higher mRNA expression level in HNSCC compared to normal head and neck tissue (P < .0001) |
| Chen, 43 2019 (China) | 167 oral SCC gene expression data set (GSE30784) and 54 oral SCC DNA methylation data set (GSE75537), obtained from the GEO | Correlations between methylation level of CpG sites and OS of oral SCC patients were assessed by univariate Cox regression analysis followed by robust likelihood‐based survival analysis | A two‐CpG‐based prognostic signature for OSCC OS prediction was obtained, which included the sites cg17892178 and cg17378966 that are located in NID2 and IDO1, respectively |
| Krishna, 39 2018 (USA) | 119 HNSCC transcriptomes | Epitope mapping from HPV+HNSCC PBMCs using Elispot, flow cytometry immune cell phenotyping, ssGSEA of HPV and immune gene signatures | IDO was strongly expressed in HPV+ vs HPV− HNSCC (P = .001). Its expression correlated with E7 HPV antigen expression (R 2 = 0.84, P = .033). Combined inhibition of PD‐1 and IDO‐1 can sensitise HPV+HNSCCs to CD8+ cytotoxic T‐lymphocyte‐mediated cytotoxicity |
| Page, 82 2018 (USA) | 3D‐EX platform, 3D tumour microspheres were produced from fresh HNSCC | NanoString analysis for expression of genes including IDO1 | Increased expression of IDO1 gene in HNSCC which were responsive to checkpoint inhibitor treatment ex vivo |
| Foy, 40 2017 (France) | 212 oral SCC who were NSND, HPV+samples were excluded. 4 cohorts: TCGA, GEO1, GEO2, CLB | Gene expression profiles generated using microarrays and targeted‐RNA sequencing. Functional pathway analysis performed using ssGSEA and STRING | IDO1 was overexpressed in tumours from NSND vs SD (P = .0046). Elderly female patients were more common in NSND and harboured less gene mutations (P = .0006). PD‐L1 and IDO1 were co‐overexpressed, suggesting a higher potential benefit of combination therapy involving both |
| Saâda‐Bouzid, 47 2017 (France) | 36 recurrent metastatic HNSCC treated with anti‐PD‐1 or anti‐PD‐L1 or in combination with a second checkpoint inhibitor | Extraction of blood DNA and genotyped by MassARRAY and multivariate analysis with PFS and OS | A genotype of IDO1 rs3739319 (A/G or A/A) was associated with a longer PFS and OS, (HR = 8.4, P = .002) and (HR = 6.2, CI95% 1.5‐25.9, P = .01), respectively. Allelic variation of IDO1 rs3739319G>A, implicated in transcription and regulation was associated with longer survival in patients treated with anti‐PD‐1 therapy |
| Wirth, 41 2017 (USA) | Validation cohort of 25 HPV+HNSCC patients | Microarray of 59 immune‐related genes to compare expression profiles in HPV+HNSCCs. qPCR and protein expression assay used in validation cohort | There was a 65‐fold increase in IDO1 in 10 PD‐L1+ vs 5 PD‐L1− HPV+HNSCCs (P = .004). IDO1 expression was upregulated and co‐localised in the TME of the validation cohort. IDO1 expression increased and correlated with disease progression in anti‐PD‐1 treated HNSCC patients |
| Won, 45 2017 (USA) | 15 patients with stage III‐IV HNSCC, blood specimen collected before, during and after RT | Gene expression analysis in patients’ PBMCs | A 3.6‐fold (P = .1) increase seen in IDO expression in patients’ PBMCs during RT along with other protein mediators that promote immune suppression, suggesting RT could induce tolerogenic effects in HNSCC which can be targeted with checkpoint inhibitor therapy |
Abbreviations: HPV, human papillomavirus; PBMCs, peripheral blood mononuclear cells; ssGSEA, single‐sample gene set enrichment analysis; SCC, squamous cell carcinoma; TCGA, The Cancer Genome Atlas; GEO, Gene Expression Omnibus; CLB, Centre Léon Bérard cancer centre, Lyon, France; NSND, never‐smokers and never‐drinkers; SD, smokers drinkers; STRING, search tool for the retrieval of interacting genes/proteins; qPCR, quantitative polymerase chain reaction; PFS, progression‐free survival; OS, overall survival; RT, radiotherapy.
3.5. Clinical trials of IDO inhibitors in HNSCC
Clinical trials with published results on IDO in HNSCC are all in early phase (I‐II) and assessed IDO1 inhibitor in combination with a PD‐1/PD‐L1 inhibitor (Table 4). All HNSCC patients enrolled in the published trials had advanced metastatic or recurrent disease. Pembrolizumab 12 , 14 , 15 , 17 (PD‐1 inhibitor) was most commonly combined with IDO inhibitors, followed by Nivolumab 16 (PD‐1 inhibitor), Durvalumab 13 and Atezolizumab 11 (both PD‐L1 inhibitors). The trials showed (a) objective responses (34%‐55%) and disease control rates (62%‐70%) for Epacadostat (IDO1 inhibitor) in combination with a PD‐1 inhibitor, 12 , 14 , 16 (b) safety profile of IDO1 and PD‐L1 inhibitor combination therapy was consistent with previous reports of each checkpoint inhibitor as monotherapies 13 and (c) IDO gene expression as a predictive biomarker for response to PD‐L1 therapy. 18 The most common treatment‐related adverse event associated with the immune checkpoint trials was fatigue (22%‐32%). 11 , 13 , 15 Existing clinical trials testing IDO inhibitors in HNSCC, registered on ClinicalTrials.gov without published results are summarised in Table S2.
TABLE 4.
Clinical trials of IDO inhibitors in HNSCC with published results
| First author, year (country) | Trial name ID | Phase | Design | Disease | Eligibility | Target(s) | Treatments | Patients (n) | Primary end point | Status | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jung, 11 2019 (South Korea) | NCT02471846 | I | Open‐label, multicentre, dose‐escalation and expansion trial | Locally advanced, recurrent or metastatic incurable solid malignancy | Progression following at least one standard therapy |
IDO1 PD‐L1 |
Navoximod Atezolizumab |
157 total 6 HNSCC |
Percentage of participants with DLTs and AEs | Completed | 75% experienced TRAEs; most common were fatigue (22%) and rash (22%). This combination demonstrated acceptable safety profile |
| Mitchell, 12 2018 (USA) |
ECHO‐202/KEYNOTE‐037 |
I/II | Multicentre, non‐randomised, open‐label trial | Advanced solid tumours: stage IIIB, IV or recurrent NSCLC, melanoma, RCC, EA, UC, TNBC or HNSCC | All patients with one or more previous therapy, or no available curative treatment |
IDO1 PD‐1 |
Epacadostat Pembrolizumab |
62 total 2 HNSCC |
Number of subjects with DLTs, ORR | Active, not recruiting |
84% experienced TRAEs, none led to death. ORR = 55% (12/22). In HNSCC, 1 CR, 1 SD. Combined safety profile similar to Pembrolizumab monotherapy |
| Naing, 13 2018 (USA) |
ECHO‐203 |
I/II | Dose‐escalation, open‐label trial | Advanced solid tumours: PC, melanoma, NSCLC, HNSCC | Failed at least 1 prior treatment, intolerant to or refused standard treatment |
IDO1 PD‐L1 |
Epacadostat Durvalumab |
34 total | Incidence of DLTs, ORR | Active, not recruiting | Most common TRAE was fatigue (32%), no TRAEs led to death. Safety profile consistent with each as monotherapy |
| Hamid, 14 2017 (USA) |
ECHO‐202/KEYNOTE‐037 |
I/II |
Multicentre, non‐randomised, open‐label trial Reporting of HNSCC results |
HNSCC | Metastatic HNSCC with ≥1 prior CT regimen |
IDO1 PD‐1 |
Epacadostat Pembrolizumab |
38 HNSCC | Number of subjects with DLTs, ORR | Active, not recruiting | ORR 34% (2 CR, 8 PR), DCR 62% (8 SD) in patients with 1‐2 prior therapies. Response observed regardless of HPV status |
| Hamid, 15 2017 (USA) |
ECHO‐202/KEYNOTE‐037 |
II |
Multicentre, non‐randomised, open‐label trial Reporting of phase II safety |
Advanced or recurrent NSCLC, melanoma, RCC, EA, UC, TNBC or HNSCC | All patients with one or more previous therapy, or no available curative treatment |
IDO1 PD‐1 |
Epacadostat Pembrolizumab |
244 total | Number of subjects with DLTs, ORR | Active, not recruiting | 55% discontinued treatment, mainly due to disease progression (n = 97). Main TRAE was fatigue (23%) |
| Perez, 16 2017 (USA) |
ECHO‐204 |
I/II | Non‐randomised, open‐label trial | Advanced cancers: melanoma, NCSCLC, HNSCC, CRC, OVC, GBM, B‐cell NHL | All adult patients with pathologically confirmed disease |
IDO1 PD‐1 |
Epacadostat Nivolumab |
241 total 36 phase I 205 phase II 23 HNSCC |
Phase I: safety and tolerability with DLTs Phase II: ORR, PFS, OS |
Active, not recruiting | Most common TRAEs: rash, fatigue, nausea, no treatment‐related deaths, 70% DCR in HNSCC |
| Gangadhar, 17 2016 (USA) |
ECHO‐202/KEYNOTE‐037 |
I | Multicentre, non‐randomised, open‐label trial | Advanced melanoma and select solid tumours | All patients with one or more previous therapy, or no available curative treatment |
IDO1 PD‐1 |
Epacadostat Pembrolizumab |
62 total 2 HNSCC |
Number of subjects with DLTs, ORR | Active, not recruiting | Reponses in 2 patients with HNSCC; 1 PR, 1 SD |
| Seiwert, 18 2016 (USA) |
KEYNOTE‐012 |
Ib | Non‐randomised, open‐label, multicentre trial | PD‐L1 positive recurrent or metastatic HNSCC | Pathologically confirmed disease, any number of prior treatment regimens | PD‐1 | Pembrolizumab |
60 total 23 HPV(+) 37 HPV(−) |
Incidence of AEs, number discontinuing due to AEs, ORR | Active, not recruiting | IDO1 found as part of six interferon‐γ related gene signature. Responders had higher IDO1 expression P = .039 |
Abbreviations: NSCLC, non‐small‐cell lung cancer; RCC, renal cell cancer; EA, endometrial adenocarcinoma; UC, urothelial carcinoma; TNBC, triple‐negative breast cancer; DLT, dose‐limiting toxicities; ORR, overall response rate; TRAE, treatment‐related adverse event; CR, complete response; PR, partial response; SD, stable disease; PC, pancreatic cancer; CT, chemotherapy; DCR, disease control rate; CRC, colorectal cancer; OVC, ovarian cancer; GBM, glioblastoma; NHL, non‐Hodgkin lymphoma; PFS, progression‐free survival; OS, overall survival; AE, adverse event.
4. DISCUSSION
4.1. Summary of main results
Existing evidence suggests that IDO is integral to TME immunity in HNSCC in the context of positive HPV status, modulating existing therapies and application in combinatorial immunotherapy. The differential expression of IDO and predicted response to immunotherapy based on simulation models as shown by cell line studies suggests that differences in IDO as a biomarker may be related to the stage of disease, site of tumour, tissue type or genomic differences between patients. These findings further support a personalised approach to future HNSCC therapies. To date, only four retrospective studies have investigated IDO expression in formalin‐fixed paraffin‐embedded tumour specimen as a prognostic biomarker for HNSCC. 35 , 36 , 37 , 38 Interestingly, Laimer 37 and colleagues showed that IDO was a prognostic factor in patients who received adjuvant (radio)chemotherapy, but had no impact in patients without adjuvant therapy. Saâda‐Bouzid 47 showed that IDO1 rs3739319 (A/G or A/A) was associated with a longer progression‐free survival and overall survival. They suggest that the prognostic and predictive value of IDO polymorphism should be tested prospectively in the context of immune checkpoint inhibitor era. Wirth and colleagues 41 showed an increase in IDO1 in PD‐L1+ HPV+HNSCCs and proposed that IDO1 is an adaptive immune resistance pathway to anti‐PD‐1 monotherapy. These results support the rationale for future combinatorial therapies involving IDO1 and PD‐1. Liang et al 30 showed that STING activation, as indicated by staining, was greatest around the nucleus in a majority (16 of 25) of HPV‐positive tongue SCC samples and was present in the whole cytoplasm in 22 of 25 HPV‐negative samples. They suggest that HPV hijacks and activates STING by DNA sensing which induces an immunosuppressive microenvironment through IDO expression and recruitment of regulatory T cells allowing the establishment of tumourigenesis in tongue SCC. The central role of IDO expression and activation by STING is also consistent with previous work on DNA sensing via STING by Huang, 48 Lemos 49 and colleagues.
4.2. IDO inhibitors commonly applied in pre‐clinical and clinical studies
IDO can be expressed in tumour cells but multiple TME cell types may also express IDO including dendritic cells, macrophages, fibroblasts, endothelial/epithelial cells and PBMCs, 50 , 51 though lymphoid cells (eg T cells and tumour‐infiltrating lymphocytes) rarely express IDO. It is important to distinguish between IDO protein abundance in the TME (detected by IHC) and IDO enzyme activity (measured peripherally in the blood and in situ using homogenised tissues), as multiple factors impact IDO activity including enzyme cofactors and natural inhibitors such as hemin and nitric oxide, respectively. As IDO inhibits innate and adaptive immunity, IDO inhibitors (IDOi) have been tested as drugs to potentiate antitumour immunity in the TME. A recent review by Lemos and colleagues summarised the IDOi under pre‐clinical and clinical evaluation. 52 In total there are seven IDOi drugs under evaluation in Phase I‐III clinical trials and four that are applied in pre‐clinical studies. The two front‐running drugs in development are the non‐selective IDOi indoximod (also known as D‐1MT or NLG‐8186) and the tryptophan competitive IDOi epacadostat (INCB024360). Drugs being tested in clinical trials include the non‐selective IDO and TDO (tryptophan 2,3‐dioxygenase) inhibitor navoximod (NLG‐919), 53 , 54 which is approximately tenfold more selective for IDO1 than TDO2; the selective IDO1 inhibitor linrodostat (BMS‐986205) 55 and IDO1 and TDO2 inhibitor PF‐06840003 56 which are both more than 100‐fold selective for IDO1 than TDO2. Indoximod, epacadostat and linrodostat are also being evaluated in combination drug trials given the strong rationale for the use of IDOi drugs as immunometabolic adjuvants to increase the efficacy of (chemo)radiotherapy and immunotherapies. 57 In vitro studies of IDOi drugs in cancer include those investigating indoximod 58 and linrodostat. 59 Applied as monotherapy to patient‐derived colorectal cancer cell lines, indoximod exhibited rather low direct cytotoxic activity, whereas coculturing the cell lines in an allogeneic setting using naïve, “unprimed” lymphocytes from healthy volunteers generally boosted the antitumoural effect of indoximod. 58 However, this was not tested in an autologous setting using partially exhausted lymphocytes from cancer patients. Interestingly, low IDO expressing cells responded better to indoximod monotherapy, suggesting that indoximod likely targets additional pathways, although the precise mechanism of action is yet to be elucidated. It is important to stress that IDOi drugs may promote antitumour effects by targeting tumour accessory cells in malignant lesions and/or in tumour‐draining lymphoid tissues, and not necessarily by targeting tumour cells per se. Nonetheless, multiple studies support the use of non‐selective IDOi drugs to counteract tumour‐induced immunosuppression and increase antitumour efficacy in combination with other therapeutics.
4.3. Other combination immunotherapeutic strategies
Apart from immune checkpoint blockade alone, other combination immunotherapeutic strategies have been studied. Rational combinations have been tested based on the knowledge that activating STING in the TME of mice stimulated protective antitumour immunity; however, preliminary outcomes from a clinical trial reveal little benefit of STING agonist monotherapy. 60 To overcome this therapy resistance, Lemos and colleagues showed that in mice bearing established Lewis lung carcinoma (LLC) tumours, intratumoural treatment with STING agonist, synthetic cyclic diadenyl monophosphate (CDA) and co‐treatment with selective COX2 inhibitor celecoxib eliminated the primary tumour burden, prevented metastases and induced durable protective antitumour immunity. 61 Co‐treatment with IDOi drugs indoximod, navoximod and linrodostat also enhanced antitumour responses to CDA, especially in co‐treatment with linrodostat which induced rapid tumour regression and increased survival, however, did not eliminate the primary tumours. Interestingly, inhibiting COX2 also significantly reduced IDO activity, which may contribute to greater antitumour activity elicited by celecoxib in combination with CDA. Another strategy to enhance antitumour immunity is to deliver recombinant enzymes that act downstream of IDO to reduce the level of immune‐suppressive Trp catabolites in the TME. PEGylated Kynureninase (KYNU) combined with immune checkpoint inhibitors or cancer vaccine reduced Kyn levels in the TME, attenuated immune suppression and promoted tumour control in vivo 62 . However, the effectiveness of this strategy in promoting clinical responses in patients is yet to be proven. Although speculative at present, combining IDOi drugs with radiotherapy treatment may modulate the TME in favour of antitumour activity and help overcome treatment resistance in cases where the disease is less radiosensitive, for instance in HPV‐negative HNSCCs where radiotherapy controls less than 50% of disease cases that have concurrent nodal metastases. The hypothesised role of IDO in the immune microenvironment is summarised in Figure 2.
FIGURE 2.
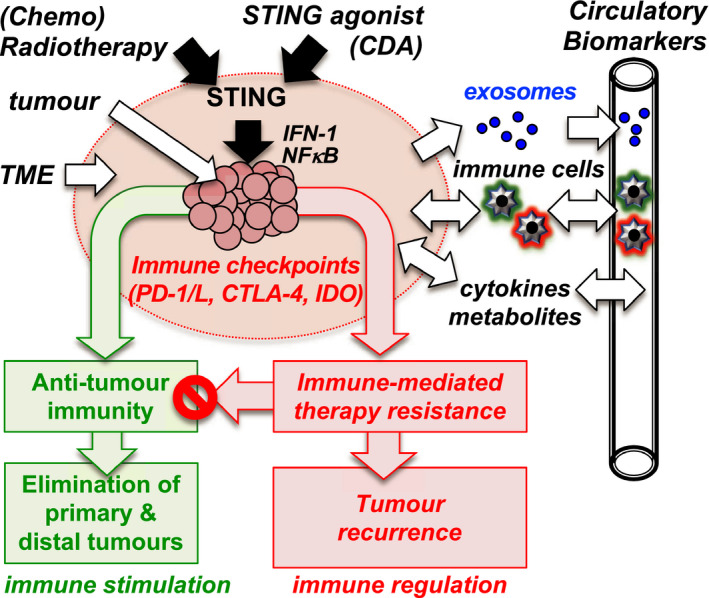
IDO immune microenvironment hypothesis. (Chemo)radiotherapy and CDA treatments activate STING to incite antitumour immunity but also boost immune regulation to enhance therapy resistance. Multiple STING‐responsive pathways involving chronic inflammation and tumour progression and associated with immune checkpoints (PD‐1/L, CTLA‐4, IDO) result in therapy resistance. Blocking these pathways modulate the TME in favour of antitumour immunity. Immune, inflammatory and metabolic biomarkers in blood reflect changes in the TME caused by treatments and therapy resistance. Abbreviations: CDA, cyclic diadenyl monophosphate; PD‐1, programmed cell death protein 1; PD‐L1, programmed death ligand 1; CTLA‐4, cytotoxic T‐lymphocyte‐associated antigen 4; IDO, indoleamine 2,3‐dioxygenase; IFN‐1, interferon‐1; NFkB, nuclear factor kappa‐light‐chain‐enhancer of activated B cells
4.4. Strengths, limitations and potential bias of evidence
This narrative systematic review synthesises the existing evidence on studies involving IDO in HNSCC. Due to the heterogeneity among the studies, a meta‐analysis was not possible and a qualitative analysis was therefore performed on the groupings of study types. Although existing HNSCC cell line studies showed IDO involvement in certain cell lines (SCC4, SCC15) and influenced by STING activation, conclusions cannot be drawn from these studies on the influence of the whole immune system or local TME dendritic cell response on the IDO pathway. Considering current published clinical trials evidence, all trials have tested an IDO1 inhibitor in combination with either a PD‐1 or PD‐L1 inhibitor. Potential reasons for a limited response seen with IDO inhibitors in existing trials could be (a) poor IDO inhibitor with a short half‐life, (b) ineffective dosing regimen and (c) redundant mechanisms and/or no synergy between PD‐1/PD‐L1 and IDO pathways. Furthermore, there has been no report of IDO immune‐based therapy or IDO inhibitor therapy in comparison with current standard of care treatments for HNSCC. In addition to IDO there are also other enzymes which have an influence on tryptophan metabolism. Apart from IDO, tryptophan 2,3‐dioxygenase (TDO2) is another rate‐limiting enzyme of the kynurenine pathway (KP). 63 TDO2 is mainly expressed in the liver; however, it has also been shown to be overexpressed in tumours as a means of immune evasion. 64 , 65 , 66 Although TDO2 and IDO activity cannot be distinguished based on peripheral blood analysis of KP activity, Riess et al 33 showed that glioblastoma multiforme (GBM) tumours had higher TDO2 expression whilst HNSCC tumours mainly presented with IDO1. While IDO is responsive to inflammatory signals (eg interferons), stress‐related signals such as glucocorticoids induce TDO2 expression, highlighting the radical differences in upstream pathways that induce KP metabolic activity. In their series an HPV‐positive HNSCC case showed the highest abundance of IDO1, reflecting its higher relative immunogenicity. In the current literature, there exists sparse evidence of the influence of TDO2 in HNSCC; however, it is possible for TDO to have an effect on the immune TME given its role in metabolic KP activity.
4.5. Implications for future clinical practice and research
Although there exists a spectrum of immune cell infiltrates in HNSCC, it is now recognised that HPV‐positive and HPV‐negative HNSCCs have distinctly different immunophenotypes characterised by increased T‐cell infiltrate, more immune cells expressing PD‐L1 and increased presence of markers of immune activation (eg granzyme and perforin) in HPV‐positive tumours whilst HPV‐negative tumours tend to have less abundant immune cell infiltrates including T cells and Tregs. 67 The immunophenotype of HNSCCs including the presence of immune cell infiltration and immune checkpoints within the TME of HPV‐positive and HPV‐negative tumour types may be important predictive biomarkers that will help guide personalised immunotherapeutic approaches in the future. A comparison of IDO1 expression data from TCGA shows that HPV‐positive HNSCCs have significantly higher IDO1 expression when compared to HPV‐negative HNSCCs (P = .001) and adjacent normal tissue (P < .001) (Figure 3) 68 although the IDO expression is also elevated in HPV‐negative HNSCC when compared to normal tissue. Furthermore, studies in various solid cancers investigating the differences between primary tumour and corresponding lymph node metastases have shown that strong tumoural IDO expression is associated with metastatic disease. 69 , 70 , 71 It has been observed in both colorectal and breast cancer that IDO expression pattern is consistent between the primary tumour and metastatic sites. 72 , 73 This suggests IDO expression as a modulator of cancer inflammation and immune evasion in both primary and metastatic tumour progression. 51 As more evidence mounts in favour of the involvement of IDO in the TME of HNSCC and a better understanding of its mechanistic role in HNSCC immune modulation emerges, IDO‐based therapies are likely to be translated to clinical practice to improve outcomes for HNSCC patients. An increasing number of new IDO inhibitors are currently being discovered. An example is DN‐016 a highly potent, selective, orally available IDO1 inhibitor with good absorption, distribution, metabolism and excretion and safety profile which was presented at ASCO 2018. 10 Also, HTI 1090 a dual inhibitor of IDO1 and hepatic enzyme tryptophan 2,3‐dioxygenase (TDO) is currently in phase I trial for advanced solid tumours including HNSCC. 74 Considered “best in class” IDO1 inhibitor, BMS‐986205 was recently halted from phase III trial 75 but earlier‐phase combination studies are still ongoing. IDO inhibitor utility as a single agent or in combination with other checkpoint inhibitors is still under clinical evaluation and recently published results show promising overall response rates in HNSCC. 12 Existing evidence suggests an increasing role for IDO‐based therapies in the context of PD‐L1 or HPV‐positive HNSCCs; however, this observation requires more mechanistic evidence to elucidate the relationship.
FIGURE 3.
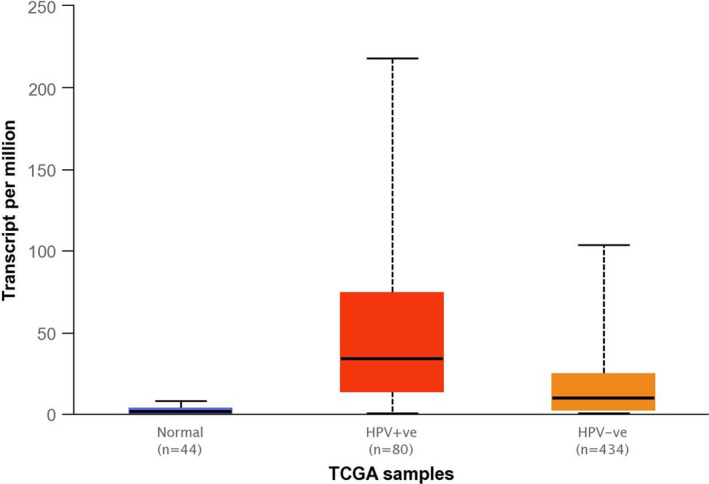
Expression of IDO1 in HNSCC based on HPV status. Box and whisker plots of IDO1 expression in HPV‐positive, HPV‐negative and normal adjacent tissue generated from TCGA data. This comparison shows significant differences in IDO1 expression when comparing: HPV‐positive vs normal tissue (P = .00002), HPV‐positive vs HPV‐negative (P = .00112) and HPV‐negative vs normal tissue (P < .00001)
Future research on IDO in HNSCC should seek to address the following questions:
Is the IDO pathway regulated by treatment (eg radiotherapy)?
Can IDO immune status be used as a predictive biomarker of treatment outcome?
What is the relationship between the IDO pathway and HPV or PD‐L1 status in HNSCC?
Improved understanding of the mechanistic role of IDO in modulating local TME immunity will inform the targeting of the IDO pathway to optimise HNSCC therapy. The ability to measure IDO activity in the peripheral blood of cancer patients 76 allows researchers to prospectively map and characterise potential groups of patients who may benefit from modified treatment doses (eg radiotherapy) based on IDO immune status. The correlation of IDO activity and expression at the tumour, draining lymph nodes, and in peripheral blood can potentially lead to less invasive sentinel lymph node and liquid biopsies to inform stratified, personalised HNSCC immune‐based therapy.
5. CONCLUSIONS
Current evidence shows the presence of IDO in the TME and suggests a link to prognosis and prediction of HNSCC treatment outcome. However, the exact mechanism of immune modulation by the IDO pathway in the TME of HNSCC remains unclear. Future translational studies need to prospectively map the activity and expression of IDO throughout HNSCC treatment to achieve a mechanistic understanding of its involvement in TME immunity and to inform the design of precision, stratified immunotherapeutic approaches involving IDO.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Table S1‐S2
Lin DJ, Ng JCK, Huang L, et al. The immunotherapeutic role of indoleamine 2,3‐dioxygenase in head and neck squamous cell carcinoma: A systematic review. Clin Otolaryngol. 2021;46:919–934. 10.1111/coa.13794
Funding information
This work was supported by the Oracle Cancer Trust Research Grant Award (BH192138); Royal College of Surgeons of England One‐Year Surgical Research Fellowship (BH180499); the Wellcome Trust Institutional Strategic Support Fund (BH182291); and the Royal College of Surgeons of Edinburgh Small Research Pump Priming Grant (BH181442).
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- 1. Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA‐4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174(3):561‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte‐associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci. 2003;100(14):8372‐8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA‐4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA‐4. Immunity. 1995;3(5):541‐547. [DOI] [PubMed] [Google Scholar]
- 4. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA‐4 blockade. Science. 1996;271(5256):1734‐1736. [DOI] [PubMed] [Google Scholar]
- 5. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus‐like autoimmune diseases by disruption of the PD‐1 gene encoding an ITIM motif‐carrying immunoreceptor. Immunity. 1999;11(2):141‐151. [DOI] [PubMed] [Google Scholar]
- 7. Okazaki T, Honjo T. PD‐1 and PD‐1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813‐824. [DOI] [PubMed] [Google Scholar]
- 8. Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of Anti–PD‐L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455‐2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dogan V, Rieckmann T, Münscher A, Busch CJ. Current studies of immunotherapy in head and neck cancer. Clin Otolaryngol. 2018;43(1):13‐21. [DOI] [PubMed] [Google Scholar]
- 10. Chen S, Liu F, Guo H, et al. Abstract 5555: discovery of DN‐016: a highly potent, selective and orally available IDO1 inhibitor for treating cancers. Cancer Res. 2018;78(13 Supplement):5555. [Google Scholar]
- 11. Jung KH, LoRusso P, Burris H, et al. Phase I study of the indoleamine 2,3‐dioxygenase 1 (IDO1) inhibitor Navoximod (GDC‐0919) administered with PD‐L1 inhibitor (Atezolizumab) in advanced solid tumors. Clin Cancer Res. 2019;25(11):3220‐3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitchell TC, Hamid O, Smith DC, et al. Epacadostat plus pembrolizumab in patients with advanced solid tumors: phase I results from a multicenter, open‐label phase I/II trial (ECHO‐202/KEYNOTE‐037). J Clin Oncol. 2018;36:3223‐3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naing A, Powderly JD, Falchook G, et al. Abstract CT177: epacadostat plus durvalumab in patients with advanced solid tumors: preliminary results of the ongoing, open‐label, phase I/II ECHO‐203 study. Cancer Res. 2018;78(13 Supplement):CT177. [Google Scholar]
- 14. Hamid O, Bauer TM, Spira AI, et al. Epacadostat plus pembrolizumab in patients with SCCHN: preliminary phase I/II results from ECHO‐202/KEYNOTE‐037. J Clin Oncol. 2017;35(15_suppl):6010. [Google Scholar]
- 15. Hamid O, Bauer TM, Spira AI, et al. Safety of epacadostat 100 mg bid plus pembrolizumab 200 mg Q3W in advanced solid tumors: phase 2 data from ECHO‐202/KEYNOTE‐037. J Clin Oncol. 2017;35(15_suppl):3012. [Google Scholar]
- 16. Perez RP, Riese MJ, Lewis KD, et al. Epacadostat plus nivolumab in patients with advanced solid tumors: preliminary phase I/II results of ECHO‐204. J Clin Oncol. 2017;35(15_suppl):3003. [Google Scholar]
- 17. Gangadhar TC, Hamid O, Smith DC, et al. Epacadostat plus pembrolizumab in patients with advanced melanoma and select solid tumors: updated phase 1 results from ECHO‐202/KEYNOTE‐037. Ann Oncol. 2016;27(suppl_6):vi380. [Google Scholar]
- 18. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐012): an open‐label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956‐965. [DOI] [PubMed] [Google Scholar]
- 19. Health and Social Care Information Centre . National Head and Neck Cancer Audit 2014 [Internet]. 2014. [cited 2016 May 26]. Available from: https://digital.nhs.uk/catalogue/PUB18081
- 20. Guo T, Califano JA. Molecular biology and immunology of head and neck cancer. Surg Oncol Clin N Am. 2015;24(3):397‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. UICC . Locally advanced squamous carcinoma of the head and neck. Rev Cancer Med WHO List Essent Med. 2014;1‐8. [Google Scholar]
- 22. Rubel F, Kern JS, Technau‐Hafsi K, et al. Indoleamine 2,3‐dioxygenase expression in primary cutaneous melanoma correlates with breslow thickness and is of significant prognostic value for progression‐free survival. J Invest Dermatol. 2018;138(3):679‐687. [DOI] [PubMed] [Google Scholar]
- 23. Okamoto A, Nikaido T, Ochiai K, et al. Indoleamine 2,3‐dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11(16):6030‐6039. [DOI] [PubMed] [Google Scholar]
- 24. Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3‐dioxygenase expression in colorectal cancer: effect on tumor‐infiltrating T cells. Clin Cancer Res. 2006;12(4):1144‐1151. [DOI] [PubMed] [Google Scholar]
- 25. Engin AB, Ozkan Y, Fuchs D, Yardim‐Akaydin S. Increased tryptophan degradation in patients with bronchus carcinoma. Eur J Cancer Care (Engl). 2010;19(6):803‐808. [DOI] [PubMed] [Google Scholar]
- 26. Suzuki Y, Suda T, Furuhashi K, et al. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer. 2010;67(3):361‐365. [DOI] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bates AM, Gomez Hernandez MP, Lanzel EA, Qian F, Brogden KA. Matrix metalloproteinase (MMP) and immunosuppressive biomarker profiles of seven head and neck squamous cell carcinoma (HNSCC) cell lines. Transl Cancer Res. 2018;7(3):533‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bates AM, Lanzel EA, Qian F, Abbasi T, Vali S, Brogden KA. Cell genomics and immunosuppressive biomarker expression influence PD‐L1 immunotherapy treatment responses in HNSCC‐a computational study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(2):157‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang D, Xiao‐Feng H, Guan‐Jun D, et al. Activated STING enhances Tregs infiltration in the HPV‐related carcinogenesis of tongue squamous cells via the c‐jun/CCL22 signal. Biochim Biophys Acta. 2015;1852(11):2494‐2503. [DOI] [PubMed] [Google Scholar]
- 31. Subramanian C, Rajendiran TM, Soni T, Cohen MS. Abstract 5481: targeting the kynurenine pathway as a novel metabolic treatment for head and neck cancer. Cancer Res. 2018;78(13 Supplement):5481.30194068 [Google Scholar]
- 32. El Jamal SM, Taylor EB, Abd Elmageed ZY, et al. Interferon gamma‐induced apoptosis of head and neck squamous cell carcinoma is connected to indoleamine‐2,3‐dioxygenase via mitochondrial and ER stress‐associated pathways. Cell Div. 2016;11(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riess C, Schneider B, Kehnscherper H, et al. Activation of the kynurenine pathway in human malignancies can be suppressed by the cyclin‐dependent kinase inhibitor dinaciclib. Front Immunol. 2020;11(February):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al‐Samadi A, Poor B, Tuomainen K, et al. In vitro humanized 3D microfluidic chip for testing personalized immunotherapeutics for head and neck cancer patients. Exp Cell Res. 2019;383(2):111508. [DOI] [PubMed] [Google Scholar]
- 35. Seppälä M, Halme E, Tiilikainen L, et al. The expression and prognostic relevance of indoleamine 2,3‐dioxygenase in tongue squamous cell carcinoma. Acta Otolaryngol. 2016;136(7):729‐735. [DOI] [PubMed] [Google Scholar]
- 36. Ye J, Liu H, Hu Y, Li P, Zhang G, Li Y. Tumoral indoleamine 2,3‐dioxygenase expression predicts poor outcome in laryngeal squamous cell carcinoma. Virchows Arch. 2013;462(1):73‐81. [DOI] [PubMed] [Google Scholar]
- 37. Laimer K, Troester B, Kloss F, et al. Expression and prognostic impact of indoleamine 2,3‐dioxygenase in oral squamous cell carcinomas. Oral Oncol. 2011;47(5):352‐357. [DOI] [PubMed] [Google Scholar]
- 38. Wang H, Mao L, Zhang T, et al. Altered expression of TIM‐3, LAG‐3, IDO, PD‐L1, and CTLA‐4 during nimotuzumab therapy correlates with responses and prognosis of oral squamous cell carcinoma patients. J Oral Pathol Med. 2019;48(8):669‐676. [DOI] [PubMed] [Google Scholar]
- 39. Krishna S, Ulrich P, Wilson E, et al. Human papilloma virus specific immunogenicity and dysfunction of CD8+ T cells in head and neck cancer. Cancer Res. 2018;78(21):6159‐6170. [DOI] [PubMed] [Google Scholar]
- 40. Foy JP, Bertolus C, Michallet MC, et al. The immune microenvironment of HPV‐negative oral squamous cell carcinoma from never‐smokers and never‐drinkers patients suggests higher clinical benefit of IDO1 and PD1/PD‐L1 blockade. Ann Oncol. 2017;28(8):1934‐1941. [DOI] [PubMed] [Google Scholar]
- 41. Wirth LJ, Burtness B, Mehra R, et al. IDO1 as a mechanism of adaptive immune resistance to anti‐PD1 monotherapy in HNSCC. J Clin Oncol. 2017;35(15_suppl):6053. [Google Scholar]
- 42. Lecerf C, Kamal M, Vacher S, et al. Immune gene expression in head and neck squamous cell carcinoma patients. Eur J Cancer. 2019;121:210‐223. [DOI] [PubMed] [Google Scholar]
- 43. Chen Y, Hei N, Zhao J, et al. A two‐CpG‐based prognostic signature for oral squamous cell carcinoma overall survival. J Cell Biochem. 2019;120(6):9082‐9090. [DOI] [PubMed] [Google Scholar]
- 44. Sailer V, Sailer U, Bawden EG, et al. DNA methylation of indoleamine 2,3‐dioxygenase 1 (IDO1) in head and neck squamous cell carcinomas correlates with IDO1 expression, HPV status, patients’ survival, immune cell infiltrates, mutational load, and interferon γ signature. EBioMedicine. 2019;48:341‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Won H, Sampath S, Massarelli E, Maghami E, Kortylewski M. Abstract 35: chemo‐radiotherapy induces tolerogenic STAT3 signaling in circulating myeloid‐derived suppressor cells in patients with head and neck squamous cell carcinoma (HNSCC). In: American Association for Cancer Research , ed. Immune Checkpoint Blockade in Cancer Therapies/Immunobiology/Targeting the Immune System. Philadelphia, Pennsylvania: American Association for Cancer Research; 2017:35. [Google Scholar]
- 46. Economopoulou P, Kladi‐Skandali A, Strati A, et al. Prognostic impact of indoleamine 2,3‐dioxygenase 1 (IDO1) mRNA expression on circulating tumour cells of patients with head and neck squamous cell carcinoma. ESMO open. 2020;5(3):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saâda‐Bouzid E, Refae S, Ebran N, et al. 31Variations in PD1, PD‐L1, IDO1 and VEGR2 genes and association with outcomes in advanced head and neck squamous cell carcinoma (HNSCC) patients treated with anti‐PD1/PD‐L1 based immunotherapy. Ann Oncol. 2017;28(suppl_7):vii12. [Google Scholar]
- 48. Huang L, Li L, Lemos H, et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J Immunol. 2013;191(7):3509‐3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lemos H, Mohamed E, Huang L, et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res. 2016;76(8):2076‐2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Campbell JD, Yau C, Bowlby R, et al. Genomic, pathway network, and immunologic features distinguishing squamous carcinomas. Cell Rep. 2018;23(1):194‐212.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meireson A, Devos M, Brochez L. IDO expression in cancer: different compartment, different functionality? Front Immunol. 2020;11:531491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lemos H, Huang L, Prendergast GC, Mellor AL. Immune control by amino acid catabolism during tumorigenesis and therapy. Nat Rev Cancer. 2019;19(3):162‐175. [DOI] [PubMed] [Google Scholar]
- 53. Mautino MR, Jaipuri FA, Waldo J, et al. Abstract 491: NLG919, a novel indoleamine‐2,3‐dioxygenase (IDO)‐pathway inhibitor drug candidate for cancer therapy. In: American Association for Cancer Research , ed. Immunology. Philadelphia, Pennsylvania: American Association for Cancer Research; 2013:491. [Google Scholar]
- 54. Nayak‐Kapoor A, Hao Z, Sadek R, et al. Phase Ia study of the indoleamine 2,3‐dioxygenase 1 (IDO1) inhibitor navoximod (GDC‐0919) in patients with recurrent advanced solid tumors. J Immunother Cancer. 2018;6(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Siu LL, Gelmon K, Chu Q, et al. Abstract CT116: BMS‐986205, an optimized indoleamine 2,3‐dioxygenase 1 (IDO1) inhibitor, is well tolerated with potent pharmacodynamic (PD) activity, alone and in combination with nivolumab (nivo) in advanced cancers in a phase 1/2a trial. In: American Association for Cancer Research , ed. Clinical Trials. Philadelphia, Pennsylvania: American Association for Cancer Research; 2017:CT116. [Google Scholar]
- 56. Reardon D, Desjardins A, Rixe O, et al. ATIM‐29. A phase 1 study of PF‐06840003, an oral indole 2,3‐dioxygenase 1 (IDO1) inhibitor in patients with malignant gliomas. Neuro Oncol. 2017;19(suppl_6):vi32. [DOI] [PubMed] [Google Scholar]
- 57. Prendergast GC, Mondal A, Dey S, Laury‐Kleintop LD, Muller AJ. Inflammatory Reprogramming with IDO1 Inhibitors: Turning Immunologically Unresponsive 'Cold' Tumors 'Hot'. Trends Cancer. 2018;4(1):38–58. 10.1016/j.trecan.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Maletzki C, Scheinpflug P, Witt A, Klar E, Linnebacher M. Targeting immune‐related molecules in cancer therapy: a comprehensive in vitro analysis on patient‐derived tumor models. Biomed Res Int. 2019;2019:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Balog A, Lin T, Maley D, Gullo‐brown J, Kandoussi EH, Zeng J. Preclinical characterization of linrodostat mesylate, a novel, potent, and selective oral indoleamine 2, 3‐dioxygenase 1 inhibitor. Mol Cancer Ther. 2020;20(3):467‐476. [DOI] [PubMed] [Google Scholar]
- 60. Harrington KJ, Brody J, Ingham M, et al. Preliminary results of the first‐in‐human (FIH) study of MK‐1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann Oncol. 2018;29:viii712. [Google Scholar]
- 61. Lemos H, Ou R, McCardle C, et al. Overcoming resistance to STING agonist therapy to incite durable protective antitumor immunity. J Immunother Cancer. 2020;8(2):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Triplett TA, Garrison KC, Marshall N, et al. Reversal of indoleamine 2,3‐dioxygenase–mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol. 2018;36(8):758‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Prendergast GC, Malachowski WJ, Mondal A, Scherle P, Muller AJ. Indoleamine 2,3‐dioxygenase and its therapeutic inhibition in cancer. Int Rev Cell Mol Biol. 2018;336:175‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Platten M, Wick W, Van Den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72(21):5435‐5440. [DOI] [PubMed] [Google Scholar]
- 65. Platten M, von Knebel DN, Oezen I, Wick W, Ochs K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol. 2015;5:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. van Baren N, Van den Eynde BJ. Tumoral immune resistance mediated by enzymes that degrade tryptophan. Cancer Immunol Res. 2015;3(9):978‐985. [DOI] [PubMed] [Google Scholar]
- 67. Solomon B, Young RJ, Rischin D. Head and neck squamous cell carcinoma: genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin Cancer Biol. 2018;52:228‐240. [DOI] [PubMed] [Google Scholar]
- 68. Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smith C, Chang MY, Parker KH, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2(8):722‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yu J, Sun J, Wang SE, et al. Upregulated expression of indoleamine 2, 3‐dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Clin Dev Immunol. 2011;2011:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Engin A, Gonul II, Engin AB, Karamercan A, Sepici Dincel A, Dursun A. Relationship between indoleamine 2,3‐dioxygenase activity and lymphatic invasion propensity of colorectal carcinoma. World J Gastroenterol. 2016;22(13):3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meireson A, Chevolet I, Hulstaert E, et al. Peritumoral endothelial indoleamine 2, 3‐dioxygenase expression is an early independent marker of disease relapse in colorectal cancer and is influenced by DNA mismatch repair profile. Oncotarget. 2018;9(38):25216‐25224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dill EA, Dillon PM, Bullock TN, Mills AM. IDO expression in breast cancer: an assessment of 281 primary and metastatic cases with comparison to PD‐L1. Mod Pathol. 2018;31(10):1513‐1522. [DOI] [PubMed] [Google Scholar]
- 74. Atridia P. A Trial of HTI‐1090 in Subjects With Advanced Solid Tumors [Internet]. ClinicalTrials.gov. 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03208959. Accessed March 31, 2021.
- 75. Bristol‐Myers S. An Immuno‐therapy Study of Nivolumab in Combination With Experimental Medication BMS‐986205 Compared to Standard of Care EXTREME Regimen in First‐line Recurrent/Metastatic Squamous Cell Carcinoma of Head and Neck [Internet]. ClinicalTrials.gov. 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03386838. Accessed March 31, 2021.
- 76. Wang W, Huang L, Jin J‐Y, et al. IDO immune status after chemoradiation may predict survival in lung cancer patients. Cancer Res. 2017;78(3):809‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med. 2012;9(5):e1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Succaria F, Kvistborg P, Engle EL, et al. Abstract 4693: characterization of the tumor immune microenvironment in head and neck squamous cell carcinoma (SCCHN). Cancer Res. 2018;78(13 Supplement):4693. [Google Scholar]
- 79. Venkata M, Saha B. 47PStudy of tumor immune contexture in patients with squamous carcinomas of head and neck in the Indian population – a pilot study. Ann Oncol. 2017;28(suppl_10):x13. [Google Scholar]
- 80. Kuales MA, Wenzel J, Schmid‐Wendtner M‐H, Bieber T, von Bubnoff D. Myeloid CD11c+ S100+ dendritic cells express indoleamine 2,3‐dioxygenase at the inflammatory border to invasive lower lip squamous cell carcinoma. Histol Histopathol. 2011;26(8):997‐1006. [DOI] [PubMed] [Google Scholar]
- 81. Ferdinande L, Deron P, Rottiers I, Bonte K, Vermeersch H, Cuvelier C. Different expression patterns of indoleamine 2,3‐dioxygenase in squamous cell carcinoma of tonsil and tongue. In: 27th International congress of the International Academy of Pathology Histopathology. 2008:212‐213.
- 82. Page MM, Varela MM, Kreahling J, Altiok S. Abstract LB‐347: integrated comprehensive analysis of immune cell subsets and assessment of checkpoint inhibitor response in head and neck squamous cell carcinoma, urothelial carcinoma and renal cell carcinoma 3D ex vivo. Cancer Res. 2018;78(13 Supplement):LB‐347. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


