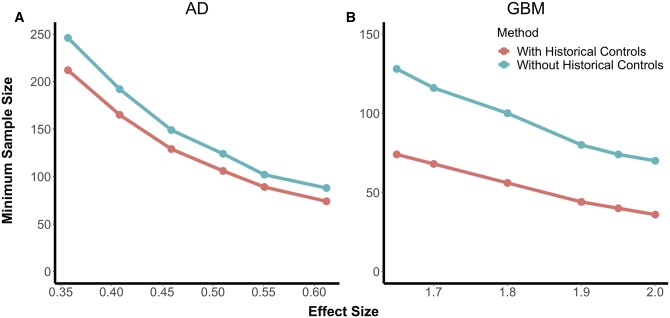
Figure 2.
Plasmode simulation results. Results from simulated studies under two scenarios. With the addition of historical controls, the minimum required sample size for 80% power is markedly lower than using classical two-sample clinical trial analysis. These figures show minimum sample size (vertical axes) required to achieve 80% power for a range of effect sizes (horizontal axes) based on observed outcome and radiomic predictions. (A) shows the results from simulations for continuous outcome measures of cognition in our Alzheimer’s cohort from ADNI, analysed using a linear regression model with and without incorporation of the radiomic predictor (left). (B) shows the results from simulations for survival in our glioblastoma cohort, comprised of 134 patients who were treated for newly diagnosed GBM at the Hospital of the University of Pennsylvania between 2006 and 2013 and analysed with an accelerated failure time model with and without incorporation of the radiomic predictor. Note that the proposed method that leverages historical controls to build radiomic predictions (red) requires lower samples sizes than the classical approach (blue). Minimum required sample size was calculated as the smallest sample size that achieved 80% power as calculated by the percentage of Monte Carlo simulations with a non-zero treatment effect that were significant at the = 0.05 level.