Figure 1. Flow diagram of inclusion and exclusion criteria for study cohort.
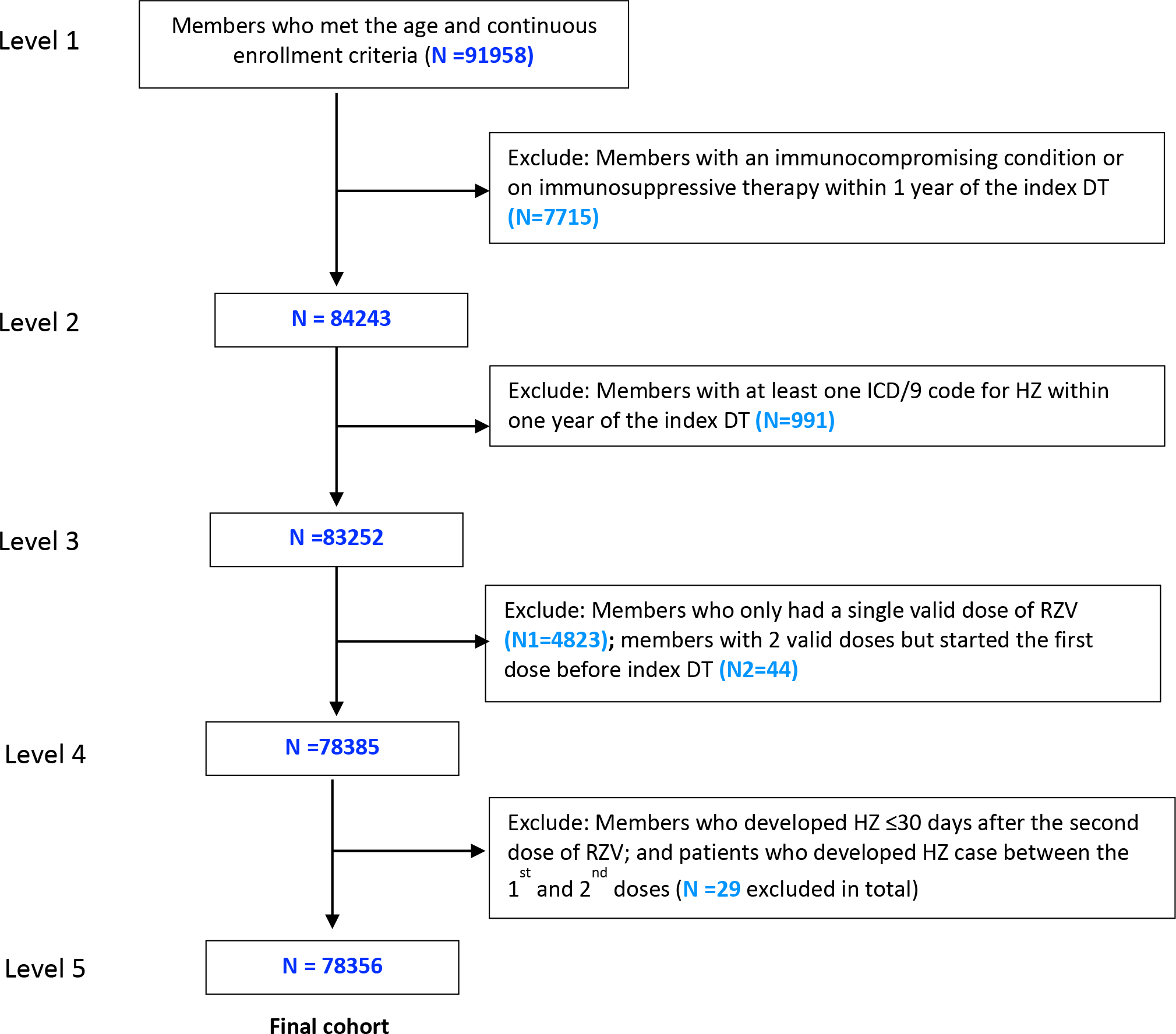
OLDW = OptumLabs Data Warehouse; ICD = International Classification of Disease; HZ = Herpes zoster; RZV = Recombinant Zoster Vaccine
a Index date was defined as the date at which an individual was eligible for study inclusion.
b Two valid doses of recombinant zoster vaccine were defined as receiving the second dose between 30 and 210 days after the first dose.
