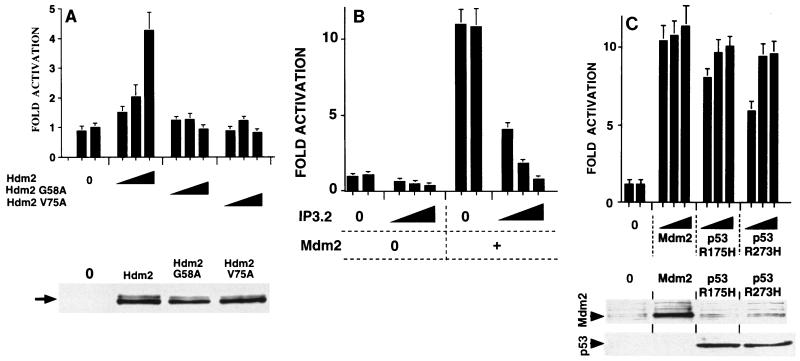
FIG. 4.
Involvement of p53 in the effects of Mdm2 on cyclin A promoter activity. tsBN462 cells were transfected in six-well dishes with the cyclin A Luc reporter (1 μg) and increasing amounts of expression vectors for Hdm2 and mutants with single-amino-acid substitutions in the p53 binding domain (0.1, 0.5, and 2 μg) (A), IP3.2 (pBC-IP3.2, 0, 0.05, 0.1 and 0.5 μg; made up to 0.5 μg with pBC) and Mdm2 (0 and 0.5 μg) (B), and Mdm2, p53 R175H, and R273H (0, 0.1, 0.5, and 2 μg) (C). After transfection the cells were transferred to 39°C, and 24 h later the luciferase activities were measured. Luciferase activities were corrected for variations in transfection efficiency by using the internal control (pSG5-LacZ; 1 μg/ml). They are presented relative to the control (set to 1) that was included in duplicate in each experiment. The fold activations are the averages from three independent experiments, and the error bars indicate the standard deviations. The Western blots in the lower panels were probed with the IF2 mouse monoclonal antibody against Mdm2 (Ab-1; Calbiochem OP46 (A) and rabbit anti-Mdm2 antibody no. 365 raised against a mouse Mdm2-derived peptide (33) and the DO1 mouse monoclonal against human p53 (C). Arrows indicate the corresponding specific bands of the expected mobility. Extracts from transfections with the highest levels of the corresponding expression vectors are shown.