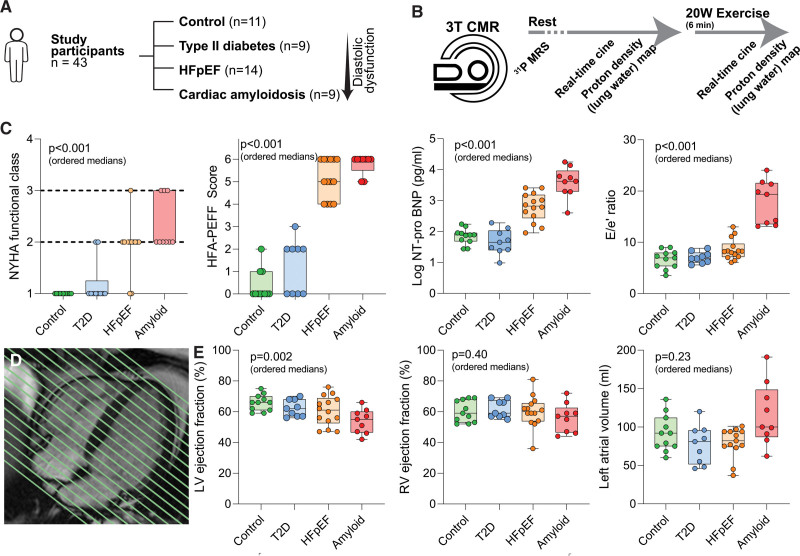
Figure 1.
Baseline clinical and cardiovascular magnetic resonance parameters. A, Participants and study groups for the basket trial design. B, Cardiovascular magnetic resonance (CMR) imaging and spectroscopy protocol at rest and during exercise using a magnetic resonance ergometer. C, Baseline clinical characteristics for New York Heart Association (NYHA) functional class, HFA-PEFF score, NT-proBNP (N-terminal pro-brain natriuretic peptide), and E/e′ ratio across the spectrum of diastolic dysfunction. D, CMR horizontal long axis image demonstrating whole-heart short axis coverage using real-time imaging. E, CMR parameters for left ventricular (LV) ejection fraction, right ventricular (RV) ejection fraction, and left atrial volume across the 4 study groups. P values (ordered medians) reported are the result of Jonckheere-Terpstra test across the groups. HFpEF indicates heart failure with preserved ejection fraction; MRS, magnetic resonance spectroscopy; and T2D, type 2 diabetes.