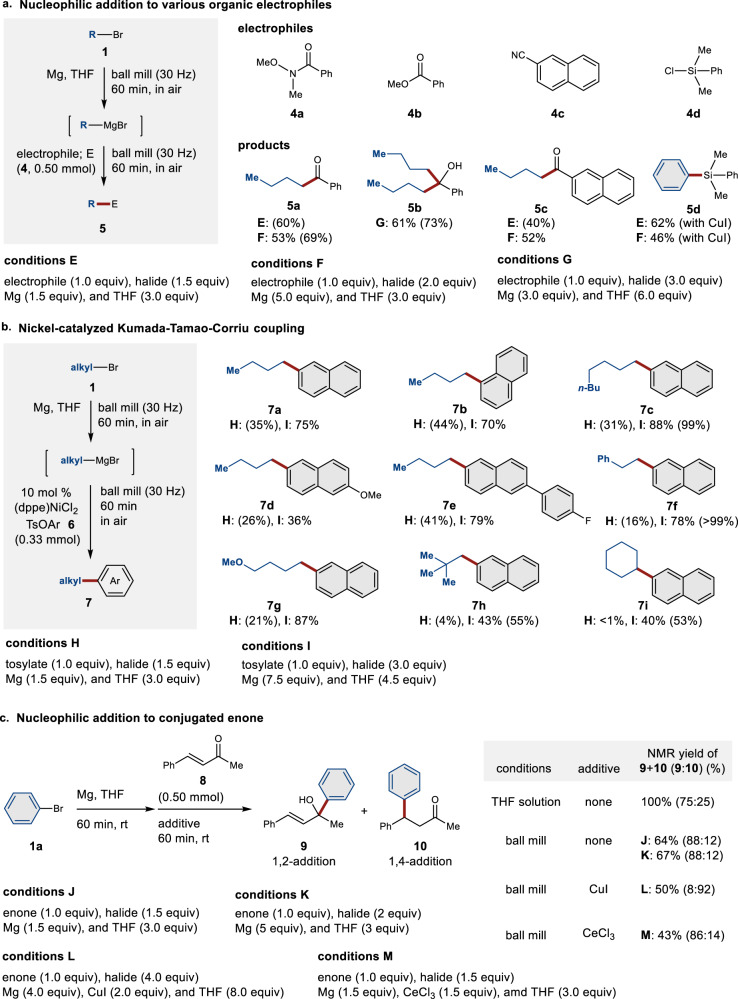
Fig. 4. Various organic transformations using mechanochemically generated organomagnesium nucleophiles.
Isolated yields are reported as percentages. Proton NMR integrated yields are shown in parentheses. a Nucleophilic addition to various electrophiles under mechanochemical conditions. b Mechanochemical nickel-catalyzed Kumada–Tamao–Corriu coupling reactions. c Nucleophilic addition reactions to conjugated enone 8 under mechanochemical conditions. A stainless-steel milling jar (5 mL) and a stainless-steel ball (diameter: 10 mm) were used. For details, see the Supplementary Information.