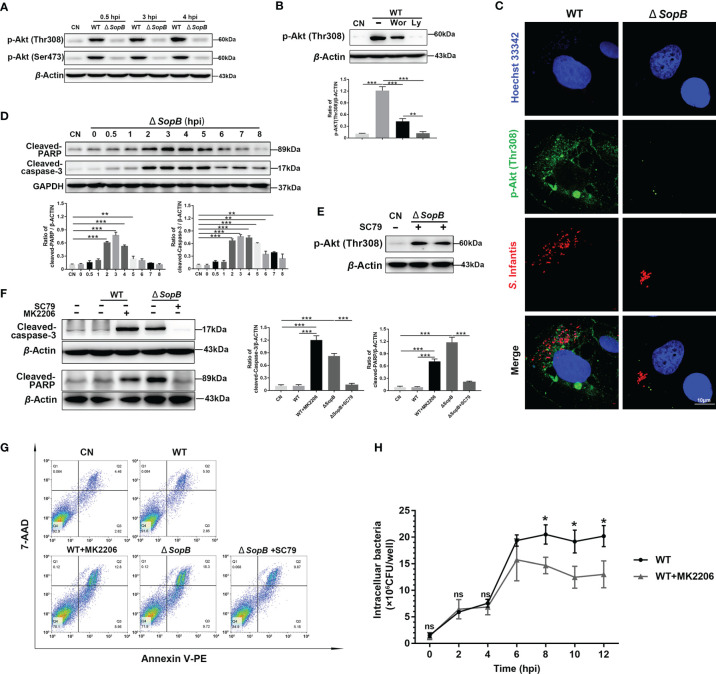
Figure 3.
Inhibition of apoptosis by intermittent Akt phosphorylation was mediated by Cytosolic S. Infantis SopB. (A) Western blot analysis of p-Akt (Ser473) and p-Akt (Thr308) protein expression levels after WT S. Infantis or SopB mutant infection at 0.5, 3, and 4 hpi. CN, control. (B) Immunoblotting verified the repressive effects of Ly294002 (Ly) and Wortmannin (Wor) on p-Akt (Thr308) protein expression levels after infection with WT S. Infantis. CN, control. (C) Immunofluorescence staining of p-Akt (Thr308) after infection with WT S. Infantis or the SopB mutant at 4 hpi. Red: S. Infantis. Green: p-Akt (Thr308). Blue: Hoechst 333342. (D) Western blot analysis of Cleaved-caspase-3 and Cleaved-PARP protein expression levels within 8 h after SopB mutant infection. CN, control. (E) Immunoblotting verified the activation of SC79 on p-Akt (Thr308) protein expression level after SopB mutant infection at 4 hpi. CN, control. (F, G) Apoptosis was evaluated after infection with WT S. Infantis or the SopB mutant at 4 hpi. In the process of bacterial infection, the phosphorylation level of Akt was regulated by the addition of MK2206 or SC79. (F) The protein levels of Cleaved-caspase-3 and Cleaved-PARP were analyzed using Western blotting. (G) The proportion of apoptotic cells was detected using flow cytometry. (H) Detection of intracellular bacterial load using the gentamicin protection assay without (total intracellular bacteria, black curve) or with (total intracellular bacteria, gray curve) MK2206. ns, no significant difference. Data were presented as the mean ± SEM from three independent experiments (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.