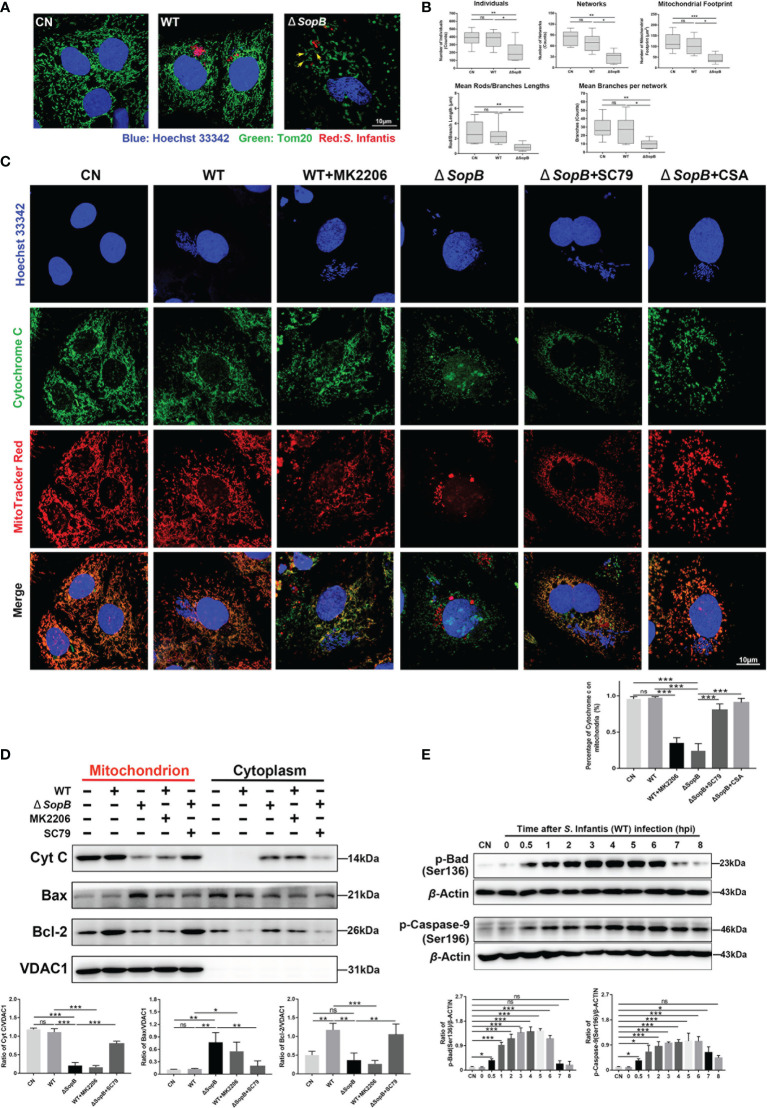
Figure 4.
SopB-mediated Akt phosphorylation inhibited apoptosis by maintaining mitochondrial dynamic network homeostasis. In (A–D), all cell samples were collected and processed at 4 hpi after infection with S. Infantis. Immunofluorescence analysis of mitochondrial network after infection with WT S. Infantis or the SopB mutant. Red: Salmonella; Green: Tom20 (mitochondria); Blue: Hoechst 333342. (B) Mitochondrial network analysis using the MiNA toolset of Image (J) (C) Co-localization of cytochrome c and mitochondria was detected using immunofluorescence. In the bacterial infection process, the phosphorylation level of Akt was regulated by the addition of MK2206 or SC79. CSA was used to inhibit the opening of the mitochondrial permeability transition pore (MPTP) Red: Mitochondria; Green: Cytochrome c; Blue: Hoechst 333342 (Nucleus and bacteria). (D) Detection of Cytochrome c, Bcl-2, and Bax protein levels in mitochondrial and cytoplasmic protein after infection with WT S. Infantis or the SopB mutant. In the bacterial infection process, the phosphorylation level of Akt was regulated by the addition of MK2206 or SC79. (E) Western blot analysis of p-Caspase-9 (Ser136) and p-Bad (Ser196) protein expression levels within 8 h after infection with S. Infantis. Data were presented as the mean ± SEM from three independent experiments (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. CN, control. ns, no significant difference.