Abstract
Background:
Ibudilast, a novel neuroimmune modulator to treat alcohol use disorder (AUD), was shown in a randomized controlled trial (NCT03489850) to reduce ventral striatum (VS) activation in response to visual alcohol cues. The present study extended this finding by probing the effects of ibudilast on alcohol cue-elicited functional connectivity (i.e., temporally-correlated activation) with the VS seed. The study also tests the association between functional connectivity and alcohol use during the trial.
Methods:
Non-treatment-seeking participants (n=45) with current alcohol use disorder were randomized to receive either ibudilast (50 mg/BID; n=20) or placebo (n=25). Upon reaching the target dose on the medication, or placebo, participants completed a functional neuroimaging alcohol cue-reactivity paradigm. Drinks per drinking day were assessed at baseline and daily during the two-week trial.
Results:
Ibudilast reduced alcohol cue-elicited functional connectivity between the VS seed and reward processing regions including the orbitofrontal and anterior cingulate cortices compared to placebo (p<0.05). Cue-elicited functional connectivity was correlated with drinks per drinking day (R2=0.5351, p<0.001), and ibudilast reduced this association in similar reward processing regions compared to placebo.
Conclusions:
Ibudilast’s effects on drinking outcomes may be related to attenuation of functional connectivity in frontostriatal circuits related to reward processing. These results provide an important proof-of-concept for this novel pharmacotherapy and support the clinical utility of incorporating neuroimaging – and especially functional connectivity – analyses into medications development.
Keywords: AUD, fMRI, Ibudilast, functional connectivity
Introduction
Alcohol use disorder (AUD) is a highly prevalent chronic relapsing disorder (Grant et al., 2015); however, it is among the most undertreated health conditions (Carvalho et al., 2019), with only 7% of adults with AUD receiving treatment (Hasin et al., 2007). The Food and Drug Administration has approved only four pharmacotherapies for the treatment of AUD to date, and these medications are limited in efficacy (Ray et al., 2019). There is a great need to develop new and more effective treatments for AUD, with a specific focus on novel molecular targets (Litten et al., 2016, 2012).
One such novel pharmacotherapy is ibudilast (IBUD; also known as MN-166, previously AV411 and available as Ketas in Japan for the treatment of bronchial asthma and for cerebrovascular disorders). Ibudilast is a selective phosphodiesterase (PDE) inhibitor (inhibiting PDE3, 4, 10, and 11) (Gibson et al., 2006) and an allosteric macrophage migration inhibitory factor (MIF) inhibitor (Cho et al., 2010), which has shown promising preclinical and clinical outcomes in the treatment of alcohol use disorder. IBUD has been shown to reduce drinking and relapse in preclinical rodent models of AUD, including preferentially reducing drinking in dependent compared to non-dependent mice (Bell et al., 2015). In a previous human laboratory study by our group, IBUD was shown to reduce craving and improve mood following stress and alcohol cue exposure (Ray et al., 2017a), but the neurobiological processes related to these clinical outcomes remain unclear.
A useful tool for identifying neural mechanisms of novel pharmacotherapies is the use of functional magnetic resonance imaging (fmri) to examine the modulation of brain activation and connectivity in regions associated with AUD (Grodin and Ray, 2019). In order to explore the mechanisms of action of IBUD in the human brain, a recent clinical trial from our group (Grodin et al., in press) employed a functional magnetic resonance imaging (fMRI) paradigm of visual alcohol cue exposure to investigate the effect of IBUD on cue-elicited neural activity in the ventral striatum (VS). This region is commonly associated with reward and has been shown to have a high expression of PDE4A, B, and D (Pérez-Torres et al., 2000) and to be highly relevant for alcohol cue-reactivity tasks (Schacht et al., 2013). The study found that IBUD significantly reduced VS activity in response to alcohol cues relative to placebo. Further, reductions in VS activity due to ibudilast were associated with reductions in drinking during the 2-week trial, as compared to placebo (Grodin et al., in press). Cue-reactivity has been shown to be predictive of treatment response (Schacht et al., 2017), demonstrating the clinical utility of functional neuroimaging in providing mechanistic data for pharmacotherapy development.
The current study is a secondary analysis of the aforementioned trial (ClinicalTrials.gov identifier: NCT03489850). While the registered aim of the main trial examined the effects of IBUD on VS cue-reactivity using an a priori defined region of interest (ROI), the current study further probes this aim and evaluates regions in which neural activation in response to alcohol cues is temporally correlated with VS cue-reactivity. This strategy, often referred to as functional connectivity (O’Reilly et al., 2012), offers a more complex, holistic picture of the circuits involved, in comparison to a single ROI (Courtney et al., 2016; Lim et al., 2019). A functional connectivity approach also builds on the main study by using the same VS region as an a priori designated seed, since its activity in response to alcohol cues was shown to be affected by IBUD in this sample (Grodin et al., in press). Previous medication studies from our group have successfully used this cue-reactivity task-based functional connectivity approach. For example, in previous studies we reported that naltrexone enhanced cue-elicited functional connectivity (relative to placebo) from a VS seed in heavy drinkers (Lim et al., 2019) and from caudate and precuneus seeds in methamphetamine users (Courtney et al., 2016).
Based on the premise that novel compounds, such as ibudilast, require proof-of-mechanism via a host of brain-based biomarkers, the primary aim of the present study was to test whether IBUD altered functional connectivity. Given that we previously found that VS cue-reactivity was significantly attenuated under IBUD, we hypothesized that VS functional connectivity would similarly be reduced in the IBUD condition compared to placebo. To connect brain-to-behavior, the current study also tested whether IBUD’s effects on functional connectivity were associated with one of the primary registered drinking outcomes of the main trial: drinks per drinking day in the week following the fMRI scan. The previous report (Grodin et al., in press) found that VS cue-reactivity predicted the drinks per drinking day outcome, such that individuals in the IBUD group who had attenuated VS activation had the fewest number of drinks per drinking day. Therefore, we hypothesized that individuals in the IBUD group who had reduced functional connectivity from the VS seed would also have the fewest drinks per drinking day. While the VS is our a priori seed of interest, we considered the broader literature on neural reactivity to alcohol cues and explored a host of additional seeds. The dorsal striatum (DS), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and precuneus were selected as alternative exploratory seeds, as these regions have shown a strong cue-reactivity signal and modulation by pharmacological and behavioral treatments (Schacht et al., 2013).
Materials and Methods
This study was performed as part of a two-week randomized controlled trial (ClinicalTrials.gov NCT03489850) of IBUD for drinking reduction. The trial was approved by the Institutional Review Board of the University of California, Los Angeles. All study participants provided written informed consent for screening, medication, and neuroimaging procedures. The current study used fMRI data from individuals who completed the neuroimaging visit.
Participants
Participants were recruited between July 2018 and March 2020 from the greater Los Angeles metropolitan area via mass transit and social media advertisements. Detailed description of the screening and experimental procedures has been published elsewhere (Grodin et al., in press). Briefly, participants included 45 non-treatment-seeking individuals with an AUD [20 IBUD, 25 Placebo; 17 female, 28 male; mean±SD age 32.51±8.59], who completed the fMRI neuroimaging paradigm after being randomly assigned to take either IBUD or placebo.
Eligibility was initially assessed through a telephone interview, after which eligible participants underwent in-person screening in the laboratory. Eligibility criteria included an age range between 21 and 50 years; meeting criteria for current AUD as assessed with the Structured Clinical Interview for DSM-5 (American Psychiatric Association, 2013) and drinking more than 14 drinks per week for men (more than 7 for women) in the 30 days prior to screening. Exclusion criteria included currently receiving or seeking treatment for AUD; past year DSM-5 diagnosis of any other substance use disorder (excluding nicotine); lifetime diagnosis of schizophrenia, bipolar disorder, or any psychotic disorder; non-removable ferromagnetic objects in body; claustrophobia; serious head injury or prolonged period of unconsciousness (>30 minutes); medical conditions thought to interfere with safe participation (unstable cardiac, renal or liver disease, uncontrolled hypertension, diabetes, or elevated liver enzymes); and pregnancy, nursing, or refusal to use reliable birth control (women). Participants were also excluded if taking medications that could interact with ibudilast or alter their alcohol use.
Participants were also assessed for a broader scope of alcohol use measures, including: a daily diary assessment, from which the study’s drinks per drinking day outcome was derived; 30-day Timeline Follow-Back (Sobell and Sobell, 1992), from which the baseline drinks per drinking day variable was derived; Structured Clinical Interview for DSM-5 (SCID) (First et al., 1995); Alcohol Use Disorder Identification Test (AUDIT) (Saunders et al., 1993); Clinical Institute Withdrawal Assessment – Alcohol Revised (CIWA-Ar) (Sullivan et al., 1989); Alcohol Dependency Scale (ADS) (Skinner and Allen, 1982); Penn Alcohol Craving Scale (PACS) (Flannery et al., 1999); and Obsessive Compulsive Drinking Scale (OCDS) (Anton, 2000). Clinical and demographic characteristics of the participants are detailed in Table 1.
Table 1.
Demographic and Clinical characteristics (collected at baseline screening visit) of ibudilast and placebo medication groups within the sample and comparisons between the groups.
| Variable | Ibudilast (n=20) | Placebo (N=25) | Comparison |
|---|---|---|---|
| Age | 34.40 ± 9.67 | 31.00 ± 7.49 |
T = −1.293 p = 0.205 |
| Gender, No. (%) | |||
| Male | 13 (65%) | 16 (64%) | X2 = 0.001 p = 0.973 |
| Female | 7 (35%) | 9 (36%) | |
| Race/Ethnicity, No. (%) | |||
| White | 14 (70%) | 11 (44%) | X2 = 8.577 p = 0.127 |
| African-American | 4 (20%) | 2 (8%) | |
| Asian | 0 (0%) | 4 (16%) | |
| Pacific Islander | 0 (0%) | 1 (4%) | |
| Mixed Race | 1 (5%) | 5 (20%) | |
| Another Race | 1 (5%) | 2 (8%) | |
| Hispanic or Latino | 5 (25%) | 6 (24%) | |
| Years of Education | 15.30 ± 2.79 | 15.28 ± 1.72 |
T = −0.075 p = 0.940 |
| Cigarette Smokers, No. (%) | 10 (50%) | 15 (60%) | X2 = 0.492 p = 0.483 |
| Baseline THC+, No. (%) | 7 (35%) | 6 (24%) | X2 = 0.228 p = 0.633 |
| AUD Severity | 0/4/9/7 | 1/6/13/5 | X2 = 1.929 p = 0.587 |
| PACS Total Score | 12.50 ± 5.52 | 11.60 ± 6.96 |
T = 0.484 p = 0.631 |
| OCDS Total Score | 19.35 ± 8.00 | 18.00 ± 9.88 |
T = 0.506 p = 0.615 |
| ADS Total Score | 13.20 ± 6.59 | 11.40 ± 6.57 |
T = 0.911 p = 0.368 |
| CIWA-Ar Total Score | 0.75 ± 1.74 | 0.44 ± 1.08 |
T = 0.695 p = 0.492 |
| AUDIT Total Score | 16.70 ± 6.30 | 16.40 ± 6.26 |
T = 0.159 p = 0.874 |
| Drinking Days (30-day baseline) | 21.25 ± 7.00 | 19.96 ± 6.30 |
T = 0.642 p = 0.525 |
| Drinks per Day (30-day baseline) | 4.02 ± 2.21 | 3.85 ± 3.77 |
T = 0.196 p = 0.846 |
| Drinks per Week (30-day baseline) | 28.19 ± 15.49 | 26.97 ± 26.41 |
T = 0.196 p = 0.846 |
| Drinks per Drinking Day (30-day baseline) | 5.91 ± 2.72 | 5.45 ± 3.85 |
T = 0.461 p = 0.647 |
At each in-person visit, participants were required to have a breath alcohol concentration (BrAC) of 0.00 g/dl and to test negative on a urine toxicology screen for all drugs of abuse (except cannabis) and urine pregnancy test (if female). IBUD was titrated as follows: 20 mg b.i.d. on days 1–2 and 50 mg b.i.d. on days 3–14. The neuroimaging session occurred at the midpoint visit, after participants had been taking medication for seven days.
fMRI Data Acquisition
Neuroimaging took place at the UCLA Center for Cognitive Neuroscience (CCN) on a 3.0T Siemens Prisma Scanner (Siemens Medical Solutions USA, Inc., Malvern, PA). A T2‐weighted, high‐resolution matched‐bandwidth (MBW) anatomical scan (time to repetition (TR) = 5,000 ms, time to echo (TE) = 34 ms, flip angle = 90°, voxel size: 1.5 mm × 1.5 × 4 mm, field of view (FOV) = 192 mm2, 34 slices, ~1.5 minutes) and a T1‐weighted magnetization‐prepared rapid gradient‐echo (MPRAGE) sequence (TR = 2,530 ms, TE = 1.74 ms, time to inversion = 1,260 ms, flip angle = 7°, voxel size: 1 mm3, FOV = 256 mm2, ~6.2 minutes) were acquired for co‐registration to the functional data. A T2*‐weighted echo planar imaging (EPI) scan (TR = 2,200 ms, TE = 35ms, flip angle = 90°, FOV = 192 mm, slices = 36, 3.0 mm, ~12 minutes) was acquired to examine the blood oxygen-level dependent (BOLD) signal during the visual alcohol cue reactivity task.
Participants completed a well-validated 720s-long visual alcohol cue-reactivity task (Schacht et al., 2013), in which they were presented with 24 pseudo-randomly interspersed blocks of alcoholic beverage images (ALC), non-alcoholic beverage images (BEV), blurred images to serve as visual controls, and a fixation cross. Each block was composed of 5 individual pictures of the same type, each presented for 4.8 seconds, for a total of 24 seconds. Each block was followed by a 6-second washout period during which participants reported on the urge to drink. Alcoholic beverage blocks were distributed between images of beer, wine, and liquor (2 of each).
Data Analysis
Preprocessing of neuroimaging data followed conventional procedures as implemented in FMRIB Software (FSL v6.0.1http://www.fmrib.ox.ac.uk/fslhttp://www.fmrib.ox.ac.uk/fsl), including motion correction (Jenkinson et al., 2002), high-pass temporal filtering (100-second cut-off), and smoothing with a 5-mm full-width, half-maximum Gaussian kernel. Functional and structural data were skull-stripped to remove non-brain tissue. Each subject’s functional images were registered to their MBW, followed by their MPRAGE using affine linear transformations, and then were normalized to the Montreal Neurological Institute (MNI) 152-brain-average template through non-linear registration (Andersson et al., 2007). All fMRI data had been used in previous studies (Burnette et al., 2021; Grodin et al., in press), and, as such, met criteria for quality control (exclusion criteria: >2mm translational displacement, >1.5° rotation). Therefore, no participants or images were excluded for quality control issues, including motion, as part of this study. The time series of activation was extracted from an a priori defined region of interest: bilateral ventral striatum (VS), a 6 mm-radius sphere centered at MNI coordinates x=12, y=6, z=9 (Schacht et al., 2017), which was then reverse-registered from standard space to each participant’s anatomical image.
Functional connectivity analyses were conducted in FSL using psychophysiological interaction (PPI) to examine the interaction of task conditions and functional connectivity between the time course of activation for specific seed regions with the rest of the brain (O’Reilly et al., 2012). PPI analyses were conducted to examine the interaction of the ALC>BEV contrast and the VS seed region for the comparisons: IBUD > PLAC and PLAC > IBUD. The first-level PPI models included four regressors: the main ‘psychological’ regressor to model the difference in task conditions (ALC-BEV), a second ‘psychological’ regressor to account for the shared variance between task conditions (ALC+BEV), a ‘physiological’ regressor to model the seed time course, and a ‘psychophysiological interaction’ regressor which is the product of the main ‘psychological’ and ‘physiological’ regressors. Age, sex, and cigarette smoking status were entered as neuroimaging-relevant covariates often associated with differential brain activation in fMRI studies. Whole-brain contrast images were generated with cluster-forming thresholds of Z>2.3 and cluster-probability thresholds of p<0.05 (Worsley, 2001). Average drinks per drinking day in the last week of the study was added as a covariate of interest in separate higher-level analyses paralleling those described above. In these analyses, baseline drinks per drinking day was also included as a covariate.
Clusters revealed by the PPI to significantly correlate with drinks per drinking day in both groups were selected as ROIs. The activation profile (percent signal change) was then extracted for these PPI ROIs using the Featquery tool in FSL for all subjects in the model, regardless of whether or not a subject had significant task-related activation in the cluster (Bradley et al., 2016). General linear model (GLM) analyses probing medication effects on the relationship between the PPI ROI activation profiles and drinks per drinking day were conducted in R (RStudio 1.2.5001), controlling for baseline drinks per drinking day. Associations between PPI ROI percent signal change and drinks per drinking day were assessed across groups, as well as separately in the IBUD and placebo groups.
Exploratory PPI analyses were also conducted to examine functional connectivity from additional seeds. The dorsal striatum (DS), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and precuneus were selected as alternative seeds (anatomical ROIs derived from the Harvard-Oxford atlas), as these regions have shown a strong cue-reactivity signal and modulation by pharmacological and behavioral treatments (Schacht et al., 2013). Age, sex, and cigarette smoking were included as neuroimaging-relevant covariates in these exploratory analyses as well.
Results
IBUD Effects on Functional Connectivity
As compared to placebo, treatment with IBUD resulted in reduced alcohol cue-elicited functional connectivity from the VS seed, as indicated by PPI analysis. Specifically, IBUD, compared to placebo, resulted in reduced functional connectivity from the VS to multiple regions including the left orbitofrontal cortex, right medial frontal cortex, and bilateral anterior cingulate (see Figure 1 and Table 2). Whole-brain results were thresholded using cluster-corrected statistics with a height-threshold of Z > 2.3 and cluster-forming threshold of p < 0.05.
Figure 1. Whole-brain analysis clusters, IBUD<PLAC, Ventral Striatum Seed.
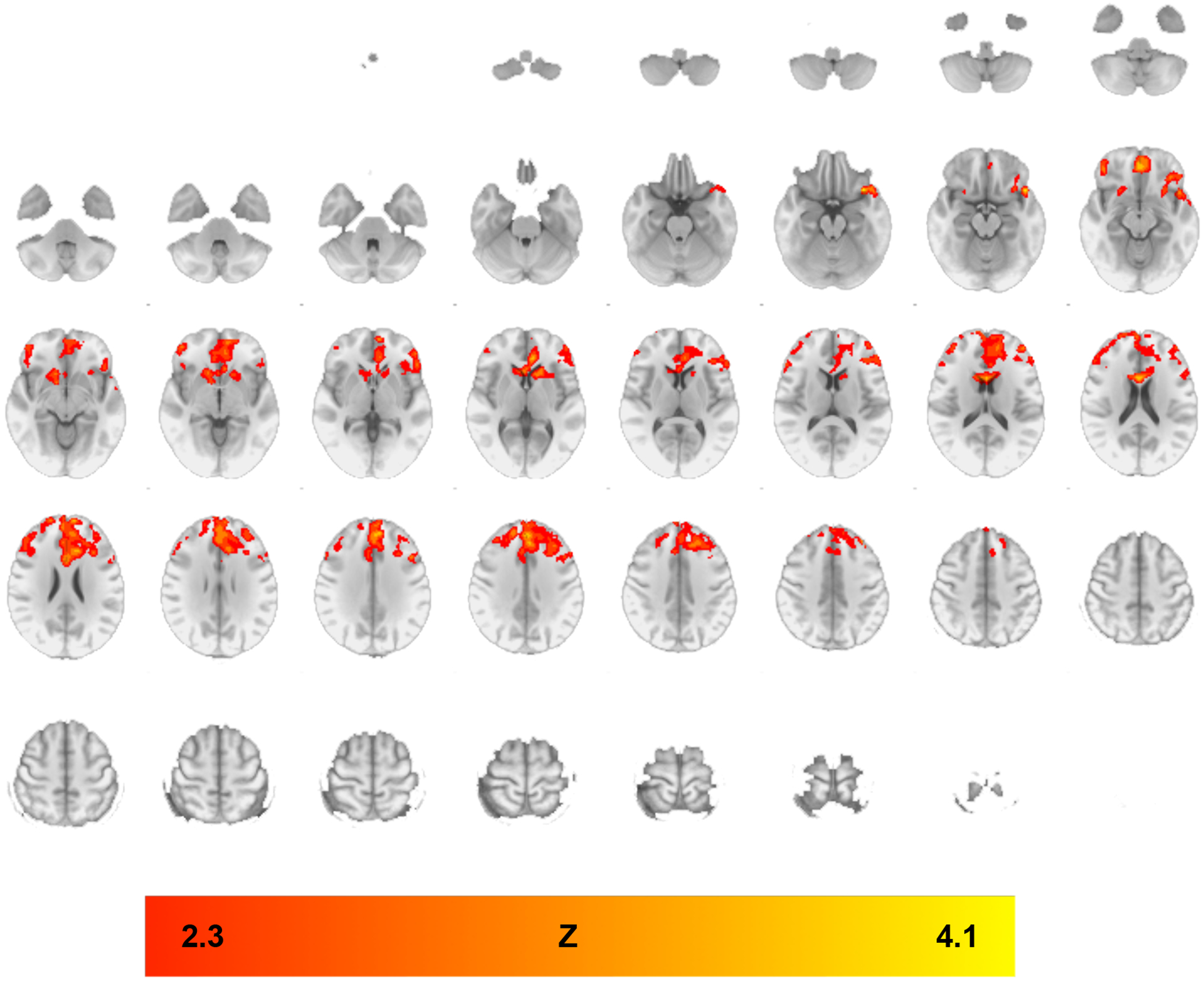
PPI analyses indicating functional connectivity from ventral striatum seed during ALC>BEV contrast in regions where functional connectivity was lower in the IBUD group than the placebo group (see Table 2 for list of clusters). Color bar represents z-values. Whole-brain results are thresholded at z > 2.3, cluster-forming threshold of p<0.05. Brain maps are displayed in radiological convention (right = left).
Table 2.
Significant clusters for psychophysiological interaction analyses using the Alcohol > Beverage contrast, IBUD<PLAC, Ventral Striatum seed. Z-statistic maps were thresholded using cluster-corrected statistics with a height-threshold of Z > 2.3 and cluster-forming threshold of p < 0.05. All coordinates are in MNI space.
| Brain Region | Cluster Voxels | Max Z-statistic | x | y | z |
|---|---|---|---|---|---|
| Placebo > Ibudilast – PPI during Alc>Bev; VS Seed | |||||
| L Orbitofrontal Cortex | 9599 | 4.13 | −46 | 18 | −16 |
| Bilateral Anterior Cingulate | 3.8 | 0 | 14 | 18 | |
| R Medial Frontal Cortex | 3.68 | 2 | 46 | −12 | |
| L Superior Frontal Gyrus | 3.65 | −6 | 46 | 32 | |
Functional Connectivity and Drinks per Drinking Day
Further PPI analysis examined the association between alcohol cue-elicited functional connectivity with the VS seed and drinks per drinking day. Whole-brain results showed that, overall (i.e., across medication groups) functional connectivity with the VS seed was correlated with drinks per drinking day and that this correlation was stronger in the placebo group than in the IBUD group. Regions in which functional connectivity showed a stronger correlation with drinks per drinking day in the placebo group compared to the IBUD group included the left caudate, temporal pole, and orbitofrontal cortex, bilateral anterior cingulate, and right lateral occipital cortex within the ALC>BEV contrast (see Figure 2 and Table 3). Whole-brain results were thresholded using cluster-corrected statistics with a height-threshold of Z > 2.3 and cluster-forming threshold of p < 0.05.
Figure 2. Drinks per Drinking Day whole-brain analysis clusters, IBUD<PLAC, Ventral Striatum Seed.
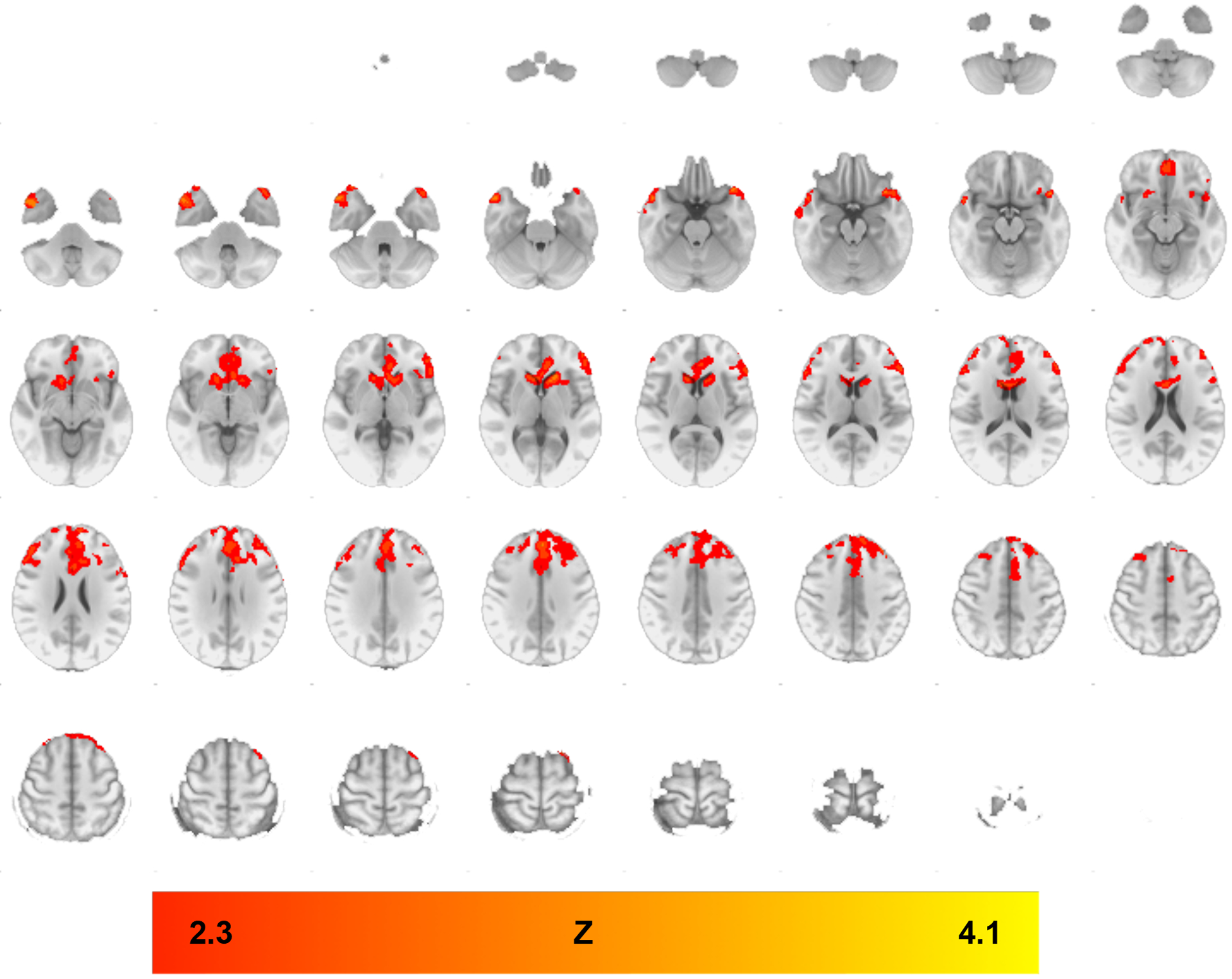
PPI analyses indicating functional connectivity from ventral striatum seed during ALC>BEV contrast with drinks per drinking day as a covariate, in regions where functional connectivity was lower in the IBUD group than the placebo group (see Table 3 for list of clusters). Color bar represents z-values. Whole-brain results are thresholded at z > 2.3, cluster-forming threshold of p<0.05. Brain maps are displayed in radiological convention (right = left).
Table 3.
Significant clusters for psychophysiological interaction analyses with drinks per drinking day as a covariate using the Alcohol > Beverage contrast, IBUD<PLAC, Ventral Striatum Seed. Z-statistic maps were thresholded using cluster-corrected statistics with a height-threshold of Z > 2.3 and cluster-forming threshold of p < 0.05. All coordinates are in MNI space.
| Brain Region | Cluster Voxels | Max Z-statistic | x | y | z |
|---|---|---|---|---|---|
| Placebo > Ibudilast – PPI during Alc>Bev × DPDD; VS Seed | |||||
| L Orbitofrontal Cortex | 8477 | 4.1 | −38 | 20 | −20 |
| L Caudate | 4.01 | −18 | 24 | 4 | |
| L Temporal Pole | 3.91 | −44 | 24 | −24 | |
| Bilateral Superior Frontal Gyrus | 3.7 | 0 | 44 | 36 | |
| Bilateral Anterior Cingulate | 3.63 | −6 | 36 | 6 | |
| R Caudate | 3.62 | 12 | 22 | 6 | |
| R Temporal Pole | 828 | 4.07 | 46 | 8 | −38 |
Across both groups, functional connectivity with the VS seed correlated positively with activation in regions including the left parahippocampal gyrus and postcentral gyrus, right frontal pole, and right inferior temporal gyrus (see Table 4). Further GLM analysis of the correlation between drinks per drinking day and connectivity between the VS seed and these regions (controlling for baseline drinks per drinking day) revealed this association to be significant across groups (R2=0.5351, p<0.001) and in the placebo group (R2=0.7363, p<0.001), but not in the IBUD group (R2=0.09506, p>0.05). These associations in the placebo and ibudilast groups were significantly different from each other (p<0.005).
Table 4.
Significant clusters for psychophysiological interaction analyses with drinks per drinking day as a covariate using the Alcohol > Beverage contrast, across groups. Z-statistic maps were thresholded using cluster-corrected statistics with a height-threshold of Z > 2.3 and cluster-forming threshold of p < 0.05. All coordinates are in MNI space.
| Brain Region | Cluster Voxels | Max Z-statistic | x | y | z |
|---|---|---|---|---|---|
| Mean functional connectivity across groups – PPI during Alc>Bev × DPDD; VS Seed | |||||
| L Parahippocampal Gyrus | 2368 | 5.12 | 22 | −16 | −26 |
| L Postcentral Gyrus | 2050 | 4.63 | −6 | −40 | 72 |
| R Frontal Pole | 1162 | 4.52 | 24 | 48 | −20 |
| R Inferior Temporal Gyrus | 952 | 4.74 | 48 | −32 | −26 |
Exploratory Analyses
PPI analyses from alternative seeds – DS, ACC, PCC, and precuneus – were conducted. Of these, only functional connectivity from the DS seed showed an effect of IBUD. Specifically, in comparison to placebo, IBUD reduced functional connectivity from the DS to the right temporal pole and middle temporal gyrus (see Figure 4 and Table 5). Whole-brain results were thresholded using cluster-corrected statistics with a height-threshold of Z > 2.3 and cluster-forming threshold of p < 0.05. This attenuation of functional connectivity from the DS seed did not show a significant correlation with drinks per drinking day.
Figure 4. Whole-brain analysis clusters, IBUD<PLAC, Dorsal Striatum Seed.
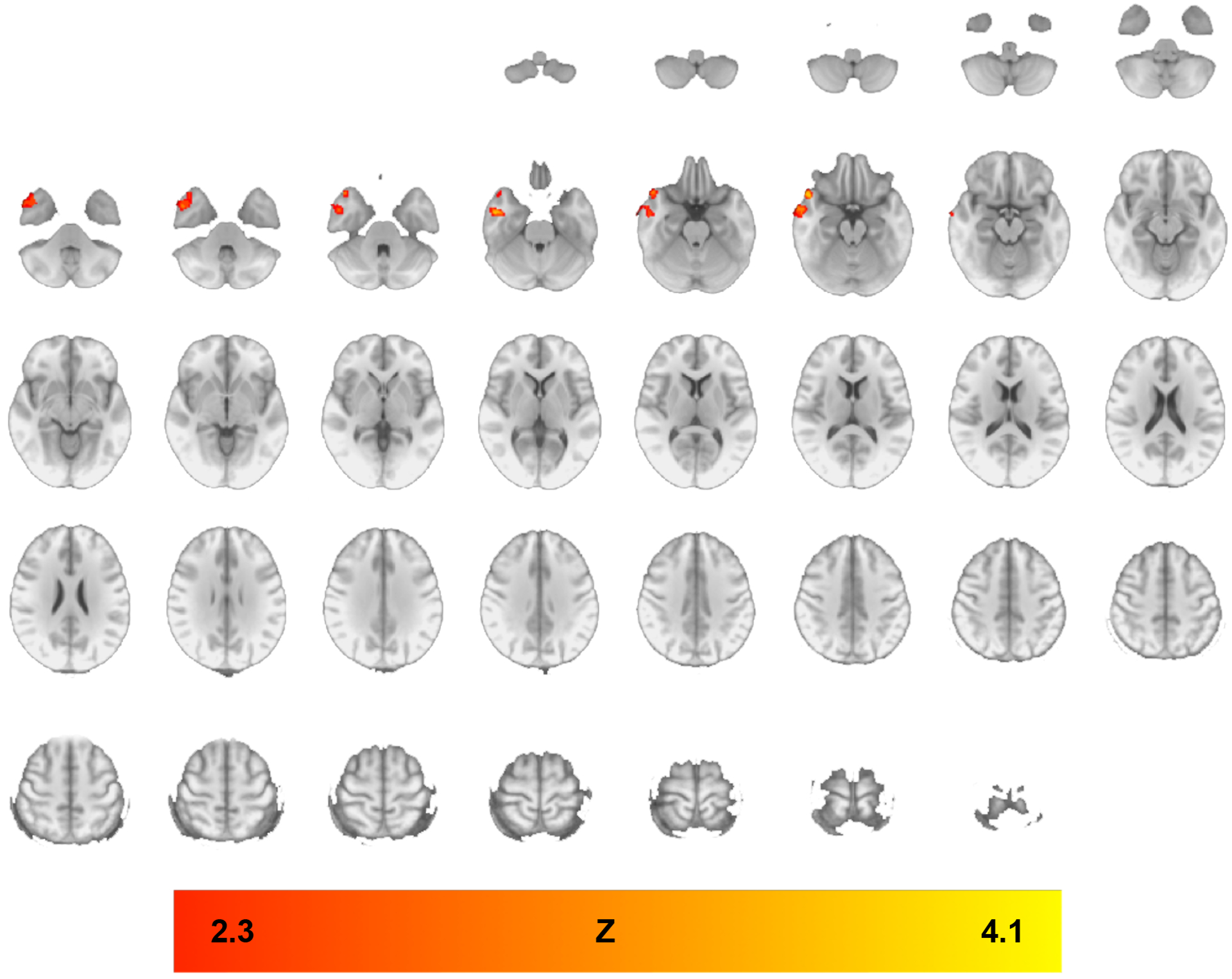
PPI analyses indicating functional connectivity from dorsal striatum seed during ALC>BEV contrast in regions where functional connectivity was lower in the IBUD group than the placebo group (see Table 5 for list of clusters). Color bar represents z-values. Whole-brain results are thresholded at z > 2.3, cluster-forming threshold of p<0.05. Brain maps are displayed in radiological convention (right = left).
Table 5.
Significant clusters for psychophysiological interaction analyses using the Alcohol > Beverage contrast, IBUD<PLAC, Dorsal Striatum seed. Z-statistic maps were thresholded using cluster-corrected statistics with a height-threshold of Z > 2.3 and cluster-forming threshold of p < 0.05. All coordinates are in MNI space.
| Brain Region | Cluster Voxels | Max Z-statistic | x | y | z |
|---|---|---|---|---|---|
| Placebo > Ibudilast – PPI during Alc>Bev; DS Seed | |||||
| R Temporal Pole | 781 | 3.68 | 58 | 12 | −42 |
| Middle Temporal Gyrus | 3.24 | 66 | 6 | −34 | |
Discussion
This study investigated the effects of ibudilast on temporally correlated activation to visual alcohol cues (i.e. cue-elicited functional connectivity) from a VS seed in a sample of individuals with current AUD. Functional connectivity analyses were employed to further our understanding of the effects of ibudilast on neural responses to alcohol cues.
Consistent with results from the main study, which found that IBUD diminished alcohol cue-reactivity in the VS ROI, IBUD was also found to reduce correlation in activity between the VS seed and frontal regions including the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC). These brain regions are heavily implicated in reward processing, decision-making, and selective attention (Volkow et al., 2011). Disrupted function in the OFC is a characteristic of addiction broadly and of AUD in particular (Moorman, 2018). Given the OFC’s primary role in controlling flexible, goal-directed behavior and its association with reward identification and acquisition, this region is implicated in regulating alcohol seeking in AUD. Of interest within the context of the current study, preclinical and clinical studies have shown cellular correlates of neuroinflammation in the OFC in both humans with AUD (Vetreno et al., 2013) and animals with chronic alcohol exposure (Qin and Crews, 2012), making this region a salient target for neuroimmune modulators like ibudilast. The ACC is strongly implicated in the experience of craving (Goldstein and Volkow, 2002), with human neuroimaging studies indicating that BOLD signal in the ACC increases in response to alcohol cues (Grüsser et al., 2004; Heinz et al., 2007). Individuals with AUD also have greater glutamate levels within the ACC than healthy controls, and ACC glutamate levels were shown to be significantly reduced by acamprosate (Frye et al., 2016; Umhau et al., 2010), another medication for AUD that may also act through neuroimmune mechanisms (Germany et al., 2018). Studies suggest that ibudilast may work similarly to protect against the hyper-glutamatergic state and maintain glutamate homeostasis in the brain (Bachtell et al., 2017; Tominaga et al., 1996). Animal (Johansson et al., 2012) and human (Pérez-Torres et al., 2000) studies show that PDE4A, B, and D are well-expressed in the cingulate and frontal cortices, indicating that IBUD may inhibit PDE throughout this reward-processing circuit. Additionally, research shows that subjective craving is correlated with alcohol cue-induced functional connectivity between the VS and regions including the OFC and ACC (Strosche et al., 2021); therefore, diminishing these connections through ibudilast may facilitate the inhibition of reward processing and craving, and ultimately a reduction in alcohol use.
To further examine this connection between brain and behavior, we conducted an exploratory analysis of associations between cue-elicited functional connectivity and drinks per drinking day in the week following the fMRI scan. Across medication groups, drinks per drinking day was positively correlated with alcohol cue-elicited functional connectivity from the VS seed. Further probing of this association revealed that brain areas in which this correlation was stronger in the placebo group than in the IBUD group included similar reward-processing regions. These results indicate that IBUD’s effects on reducing functional connectivity were indeed beneficial, as it also reduced drinks per drinking day during the two-week trial. Additionally, recency of drinking was considered as a variable. The IBUD and placebo groups did not differ significantly on recency of drinking (p>0.05). When included as a covariate in the models, recency of drinking did not significantly impact the results, and therefore was not included in the final model.
In order to probe the specificity of the effects seen from the VS seed, we conducted exploratory PPI analyses from alternative seeds, including the DS, ACC, PCC, and precuneus. Of these exploratory analyses, only the DS seed showed an IBUD-associated reduction in functional connectivity, and did not predict drinks per drinking day. These results indicate that the findings from the VS seed were relatively specific, especially those that correlated with drinks per drinking day. However, it is worth noting that IBUD’s effects on alcohol-induced functional connectivity from the VS may not be the only mechanism underlying the effects of IBUD on drinking outcomes.
This study has several strengths and limitations that should be considered in evaluating its findings. It is strengthened by the combination of neurobiological and self-reported behavioral (real-world drinking) outcomes. Another strength lies in the utilization of psychophysiological interaction (PPI) analyses to explore functional connectivity, allowing us to visualize effects of IBUD on broader reward processing circuitry beyond the ventral striatum itself. However, as mentioned in the main paper, the study is limited by its modest neuroimaging sample size, as well as its recruitment of a non-treatment-seeking sample, meaning that these results may not generalize to a sample of treatment-seeking participants (Ray et al., 2017b). An ongoing randomized controlled trial of IBUD (NCT03594435) aims to expand these results to a larger sample of treatment-seeking individuals with AUD. Additionally, IBUD’s actions on PDE have the potential to result in vascular effects. In order to probe these effects, blood pressure was collected at every in-person visit. However, the IBUD and placebo groups did not differ significantly on systolic or diastolic blood pressure at either baseline or at the scan visit, nor did either group’s blood pressure at the scan visit differ significantly from their baseline blood pressure (p>0.05). Finally, this study was conducted in a relatively high-functioning outpatient sample with mild-to-severe AUD, which may have limited our ability to detect effects of IBUD on pathologies associated with greater AUD severity levels. The ongoing trial in treatment-seekers may serve to address this outstanding question as well, as treatment-seeking populations tend to report a greater number of AUD symptoms and consume more drinks per drinking day (Ray et al., 2017b). Severity of AUD and overall brain pathology may be particularly relevant given that ibudilast has been studied for a host of brain-based biomarkers in clinical trials for multiple sclerosis (Fox et al., 2018; Naismith et al., 2021).
Ibudilast’s effects are hypothesized to be mediated by its effects on neuroinflammation and brain volume and structural integrity (Mizuno et al., 2004). Therefore, while beyond the scope of the current paper, future studies associating neural effects of ibudilast with inflammatory markers, as well as work probing possible long-term effects of ibudilast on brain morphometry, are warranted.
The use of neuroimaging in medications development continues to evolve (Grodin and Ray, 2019). Understanding the neurobiological mechanisms of action of novel pharmacotherapies represents an important aspect of medications development, especially when these biological findings are paired with disorder-related behavioral outcomes (i.e. drinking outcomes as in the case of the current study), representing a window into the clinical utility of neuroimaging in the development of pharmacological treatments. This study’s combination of a functional connectivity analysis based on an original a priori ROI analysis extends previous research to explore the actions of IBUD beyond a single region, showing its attenuation of functional connectivity throughout a reward-processing circuit including the ventral striatum, orbitofrontal cortex, and anterior cingulate cortex. Furthermore, the current study supports the primary neuroimaging finding from the main clinical trial – i.e. that IBUD diminishes VS reactivity to visual alcohol cues and that this effect is associated with drinking outcomes – and expands on these findings to give an early proof of mechanism that IBUD’s effects on drinking outcomes may be specifically related to effects in broader frontostriatal neural circuitry related to reward processing.
Figure 3. Correlation between functional connectivity from Ventral Striatum seed and Drinks per Drinking Day.
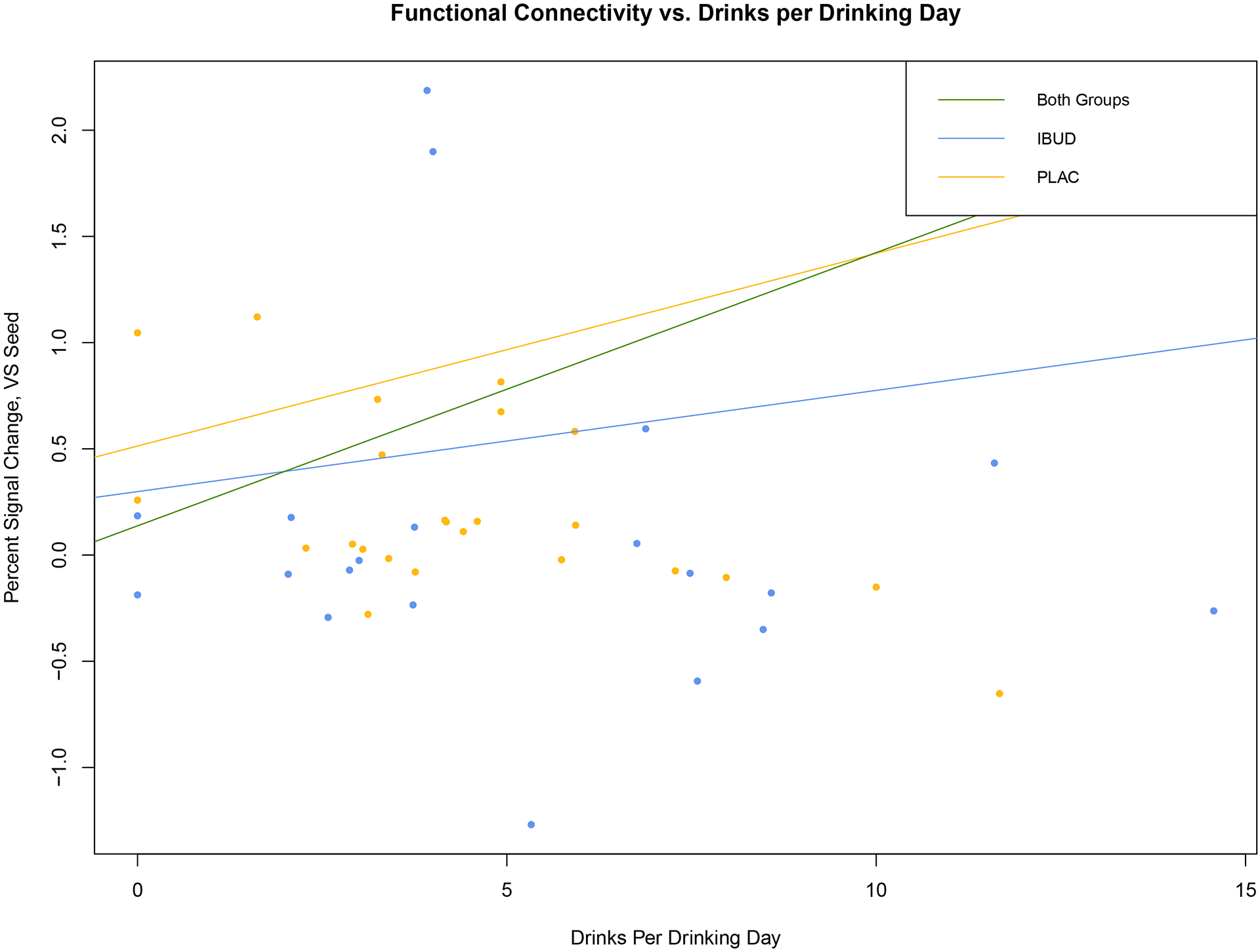
Activation profile (percent signal change) within clusters showing correlated activation from VS seed vs. Drinks per Drinking Day in the last week of the trial (controlling for baseline drinks per drinking day. Across groups (green): R2=0.5351, p<0.001; IBUD (blue): R2=0.09506, p>0.05: Placebo (yellow): R2=0.7363, p<0.001.
Funding and Disclosures
This study was supported in part by the National Institute of Drug Abuse (P50 DA005010-33 [PI: CE; Pilot Project PI: LAR]) and the National Institute of Alcohol Abuse and Alcoholism (K24AA025704 to LAR; F32AA027699 to ENG; F31AA028976 to EMB). Study medication was provided by MediciNova. The authors declare that they have no conflict of interest.
References
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. ed. American Psychiatric Association. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Andersson J, Jenkinson M, Smith S, 2007. Non-Linear Registration, aka Spatial Normalisation: FMRIB Technical Report. Oxford University, Oxford, England. [Google Scholar]
- Anton RF, 2000. Obsessive-compulsive aspects of craving: development of the obsessive compulsive drinking scale. Addiction 95, 211–217. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Jones JD, Heinzerling KG, Beardsley PM, Comer SD, 2017. Glial and neuroinflammatory targets for treating substance use disorders. Drug Alcohol Depend 180, 156–170. 10.1016/j.drugalcdep.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Lopez MF, Cui C, Egli M, Johnson KW, Franklin KM, Becker HC, 2015. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol 20, 38–42. 10.1111/adb.12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KAL, Colcombe S, Henderson SE, Alonso CM, Milham MP, Gabbay V, 2016. Neural correlates of self-perceptions in adolescents with major depressive disorder. Developmental Cognitive Neuroscience 19, 87–97. 10.1016/j.dcn.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette EM, Grodin EN, Schacht JP, Ray LA, 2021. Clinical and Neural Correlates of Reward and Relief Drinking. Alcohol Clin Exp Res 45, 194–203. 10.1111/acer.14495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Heilig M, Perez A, Probst C, Rehm J, 2019. Alcohol use disorders. Lancet 394, 781–792. 10.1016/S0140-6736(19)31775-1 [DOI] [PubMed] [Google Scholar]
- Cho Y, Crichlow GV, Vermeire JJ, Leng L, Du X, Hodsdon ME, Bucala R, Cappello M, Gross M, Gaeta F, Johnson K, Lolis EJ, 2010. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc Natl Acad Sci U S A 107, 11313–11318. 10.1073/pnas.1002716107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchison K, Roche DJO, Ray LA, 2016. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol 21, 3–22. 10.1111/adb.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, et al. , 1995. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II): II. Multi-site test-retest reliability study. Journal of Personality Disorders 9, 92–104. 10.1521/pedi.1995.9.2.92 [DOI] [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM, 1999. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol. Clin. Exp. Res 23, 1289–1295. [PubMed] [Google Scholar]
- Fox RJ, Coffey CS, Conwit R, Cudkowicz ME, Gleason T, Goodman A, Klawiter EC, Matsuda K, McGovern M, Naismith RT, Ashokkumar A, Barnes J, Ecklund D, Klingner E, Koepp M, Long JD, Natarajan S, Thornell B, Yankey J, Bermel RA, Debbins JP, Huang X, Jagodnik P, Lowe MJ, Nakamura K, Narayanan S, Sakaie KE, Thoomukuntla B, Zhou X, Krieger S, Alvarez E, Apperson M, Bashir K, Cohen BA, Coyle PK, Delgado S, Dewitt LD, Flores A, Giesser BS, Goldman MD, Jubelt B, Lava N, Lynch SG, Moses H, Ontaneda D, Perumal JS, Racke M, Repovic P, Riley CS, Severson C, Shinnar S, Suski V, Weinstock-Guttman B, Yadav V, Zabeti A, NN102/SPRINT-MS Trial Investigators, 2018. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N Engl J Med 379, 846–855. 10.1056/NEJMoa1803583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye MA, Hinton DJ, Karpyak VM, Biernacka JM, Gunderson LJ, Feeder SE, Choi D-S, Port JD, 2016. Anterior cingulate glutamate is reduced by acamprosate treatment in patients with alcohol dependence. J Clin Psychopharmacol 36, 669–674. 10.1097/JCP.0000000000000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germany CE, Reker AN, Hinton DJ, Oliveros A, Shen X, Andres-Beck LG, Wininger KM, Trutchul M, Cvek U, Choi D-S, Nam HW, 2018. Pharmacoproteomics Profile in Response to Acamprosate Treatment of an Alcoholism Animal Model. Proteomics 18, e1700417. 10.1002/pmic.201700417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LCD, Hastings SF, McPhee I, Clayton RA, Darroch CE, Mackenzie A, Mackenzie FL, Nagasawa M, Stevens PA, Mackenzie SJ, 2006. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur J Pharmacol 538, 39–42. 10.1016/j.ejphar.2006.02.053 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2002. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159, 1642–1652. 10.1176/appi.ajp.159.10.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS, 2015. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72, 757–766. 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Bujarski S, Towns B, Burnette E, Nieto S, Lim A, Lin J, Miotto K, Gillis A, Irwin M, Evans C, Ray LA, in press. Ibudilast for the treatment of Alcohol Use Disorder: A randomized placebo-controlled experimental study. Translational Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Ray LA, 2019. The Use of Functional Magnetic Resonance Imaging to Test Pharmacotherapies for Alcohol Use Disorder: A Systematic Review. Alcohol Clin Exp Res 43, 2038–2056. 10.1111/acer.14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A, 2004. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl.) 175, 296–302. 10.1007/s00213-004-1828-4 [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF, 2007. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 64, 830–842. 10.1001/archpsyc.64.7.830 [DOI] [PubMed] [Google Scholar]
- Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grüsser SM, Kienast T, Smolka MN, Flor H, Mann K, 2007. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol Clin Exp Res 31, 1138–1147. 10.1111/j.1530-0277.2007.00406.x [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- Johansson EM, Reyes-Irisarri E, Mengod G, 2012. Comparison of cAMP-specific phosphodiesterase mRNAs distribution in mouse and rat brain. Neurosci Lett 525, 1–6. 10.1016/j.neulet.2012.07.050 [DOI] [PubMed] [Google Scholar]
- Lim AC, Ghahremani DG, Grodin EN, Green R, Bujarski S, Hartwell EE, Courtney KE, Hutchison K, Miotto K, Ray LA, 2019. Neuroimaging Findings from an Experimental Pharmacology Trial of Naltrexone in Heavy Drinkers of East Asian Descent. Drug Alcohol Depend 200, 181–190. 10.1016/j.drugalcdep.2019.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A, 2012. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol 17, 513–527. 10.1111/j.1369-1600.2012.00454.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Falk DE, Ryan ML, Fertig JB, 2016. Discovery, Development, and Adoption of Medications to Treat Alcohol Use Disorder: Goals for the Phases of Medications Development. Alcohol. Clin. Exp. Res 40, 1368–1379. 10.1111/acer.13093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J, Harris RA, 2017. The Neuroimmune Basis of Excessive Alcohol Consumption. Neuropsychopharmacology 42, 376. 10.1038/npp.2016.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic V, Ansell E, Simpson C, Siedlarz KM, Sinha R, Fox HC, 2017. Peripheral Immune System Adaptations and Motivation for Alcohol in Non-Dependent Problem Drinkers. Alcohol Clin Exp Res 41, 585–595. 10.1111/acer.13317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Kurotani T, Komatsu Y, Kawanokuchi J, Kato H, Mitsuma N, Suzumura A, 2004. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology 46, 404–411. 10.1016/j.neuropharm.2003.09.009 [DOI] [PubMed] [Google Scholar]
- Moorman DE, 2018. The role of the orbitofrontal cortex in alcohol use, abuse, and dependence. Prog Neuropsychopharmacol Biol Psychiatry 87, 85–107. 10.1016/j.pnpbp.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith RT, Bermel RA, Coffey CS, Goodman AD, Fedler J, Kearney M, Klawiter EC, Nakamura K, Narayanan S, Goebel C, Yankey J, Klingner E, Fox RJ, SPRINT-MS investigators, 2021. Effects of Ibudilast on MRI Measures in the Phase 2 SPRINT-MS Study. Neurology 96, e491–e500. 10.1212/WNL.0000000000011314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, Johansen-Berg H, 2012. Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci 7, 604–609. 10.1093/scan/nss055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Torres S, Miró X, Palacios JM, Cortés R, Puigdoménech P, Mengod G, 2000. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat 20, 349–374. 10.1016/s0891-0618(00)00097-1 [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT, 2012. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation 9, 130. 10.1186/1742-2094-9-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Grodin E, Hartwell E, Green R, Venegas A, Lim AC, Gillis A, Miotto K, 2019. State-of-the-Art Behavioral and Pharmacological Treatments for Alcohol Use Disorder. Am J Drug Alcohol Abuse 45, 124–140. 10.1080/00952990.2018.1528265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Shoptaw S, Roche DJ, Heinzerling K, Miotto K, 2017a. Development of the Neuroimmune Modulator Ibudilast for the Treatment of Alcoholism: A Randomized, Placebo-Controlled, Human Laboratory Trial. Neuropsychopharmacology 42, 1776–1788. 10.1038/npp.2017.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Yardley MM, Roche DJO, Hartwell EE, 2017b. Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Abuse 43, 703–710. 10.1080/00952990.2017.1312423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, Fuente JRDL, Grant M, 1993. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 88, 791–804. 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H, 2013. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addiction Biology 18, 121–133. 10.1111/j.1369-1600.2012.00464.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Randall PK, Latham PK, Voronin KE, Book SW, Myrick H, Anton RF, 2017. Predictors of Naltrexone Response in a Randomized Trial: Reward-Related Brain Activation, OPRM1 Genotype, and Smoking Status. Neuropsychopharmacology 42, 2640–2653. 10.1038/npp.2017.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA, 1982. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol 91, 199–209. 10.1037//0021-843x.91.3.199 [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline Follow-Back, in: Litten RZ, Allen JP (Eds.), Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press, Totowa, NJ, pp. 41–72. 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- Strosche A, Zhang X, Kirsch M, Hermann D, Ende G, Kiefer F, Vollstädt-Klein S, 2021. Investigation of brain functional connectivity to assess cognitive control over cue-processing in Alcohol Use Disorder. Addict Biol 26, e12863. 10.1111/adb.12863 [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM, 1989. Assessment of Alcohol Withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). British Journal of Addiction 84, 1353–1357. 10.1111/j.1360-0443.1989.tb00737.x [DOI] [PubMed] [Google Scholar]
- Tominaga Y, Nakamura Y, Tsuji K, Shibata T, Kataoka K, 1996. Ibudilast protects against neuronal damage induced by glutamate in cultured hippocampal neurons. Clin Exp Pharmacol Physiol 23, 519–523. 10.1111/j.1440-1681.1996.tb02772.x [DOI] [PubMed] [Google Scholar]
- Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, Adams LJ, Vengeliene V, Spanagel R, Zhang Y, Shen J, George DT, Hommer D, Heilig M, 2010. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry 67, 1069–1077. 10.1001/archgenpsychiatry.2010.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Qin L, Crews FT, 2013. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiol Dis 59, 52–62. 10.1016/j.nbd.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F, 2011. Addiction: Beyond dopamine reward circuitry. Proc Natl Acad Sci U S A 108, 15037–15042. 10.1073/pnas.1010654108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, 2001. Statistical Analysis of activation images. Funct MRI 14, 251–270. [Google Scholar]


