Fig. 5.
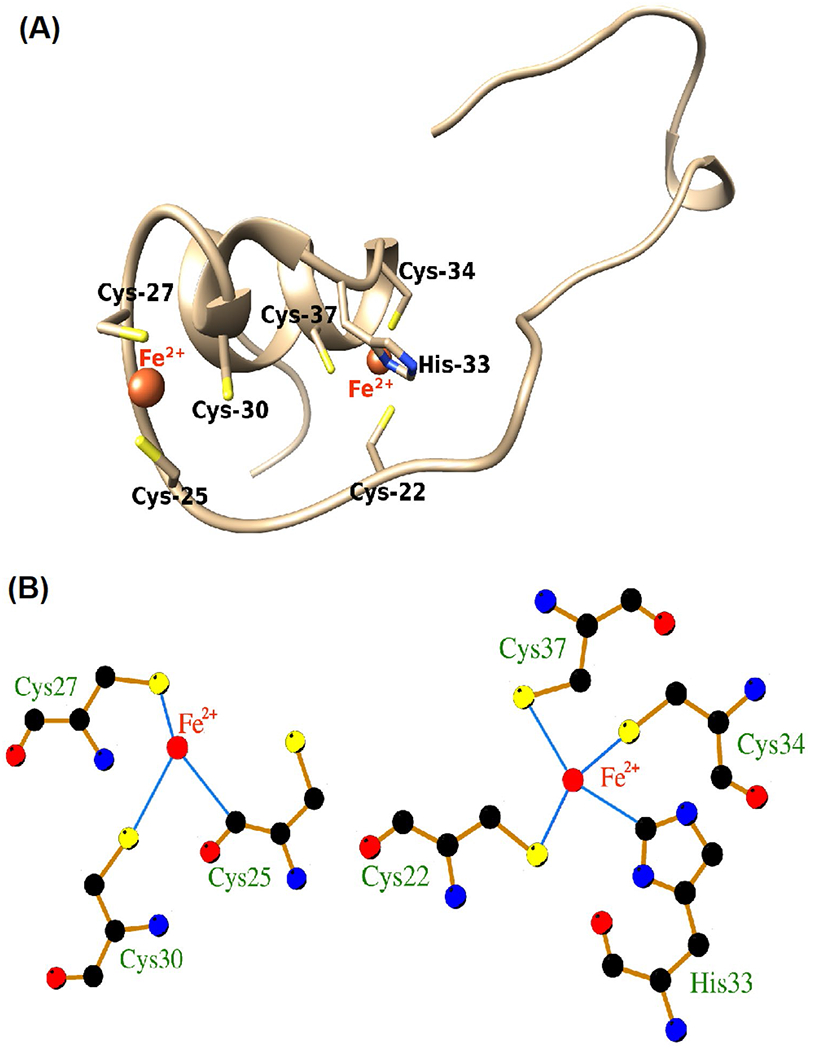
Computer modeling of iron-binding sites on HIV-1 Tat protein. Computer modeling indicated that the cysteine-rich domain of HIV-1 Tat protein (Cys-22, Cys-25, Cys-27, Cys-30, His-33, Cys-34, Cys-37) is capable of interacting with ferrous (Fe2+) and ferric (Fe3+) iron. A Molecular interaction modeling predicted that Fe2+ interacted at two sites of Tat22-37; both involve the cysteine-rich helical region of the protein involving 7 amino acid residues (Cys-22, Cys-25, Cys-27, Cys-30, His-33, Cys-34, Cys-37). B At one site, Fe2+ formed three interaction bonds with sulfhydryl (–SH) groups on Cys-25, Cys-27, and Cys-30. At other sites, Fe2+ formed four interaction bonds with –SH groups on Cys-22, Cys-34, and Cys-37 and the N-atom of the histidine imidazole ring on His-33. Illustrated are Fe2+ and Fe3+ (red), hydrogen bonds (green), bond lengths, and coordinate bonds (thin blue lines) (http://bioinfo.cmu.edu.tw/MIB/)
