Fig. 7.
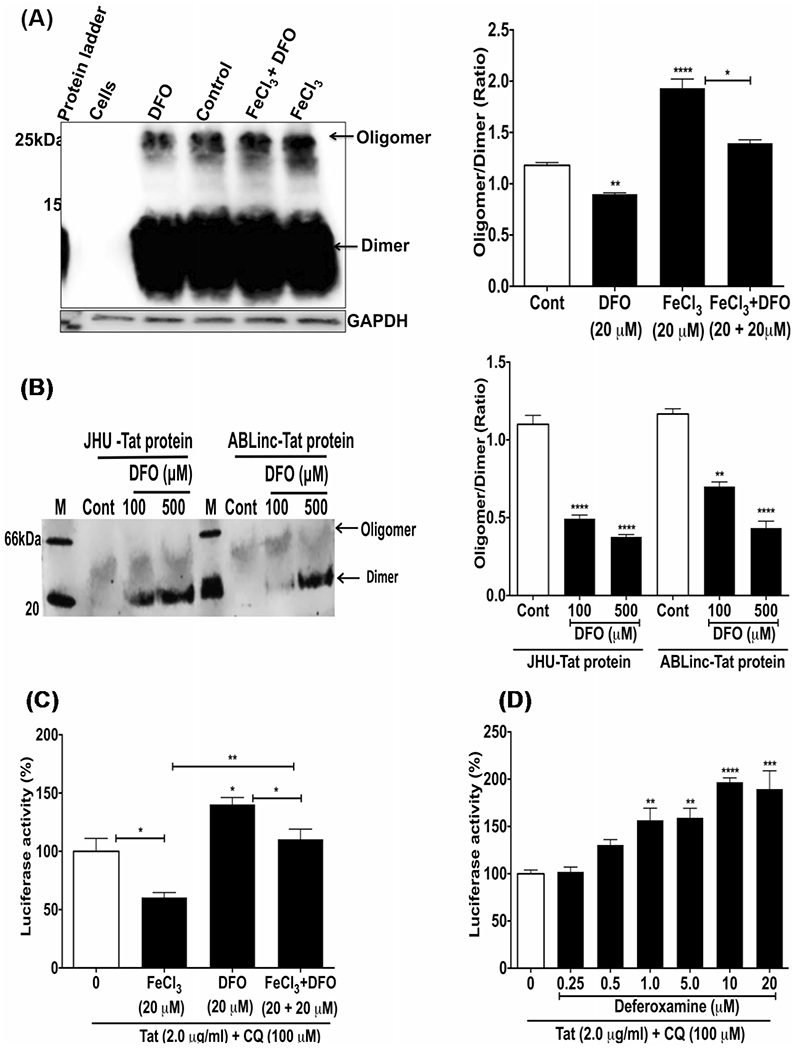
Deferoxamine (DFO) prevented and reversed FeCl3-induced HIV-1 Tat oligomerization, and increased Tat-mediated HIV-1 LTR transactivation. A Tat protein (7.0 μg/ml) was incubated for 6 h at 37 °C with U87MG cells alone and in combination with 20 μM FeCl3, 20 μM DFO, and 20 μM FeCl3 plus 20 μM DFO. Immunoblots for HIV-1 Tat using 15% SDS-PAGE showed that DFO decreased significantly (p < 0.01) Tat oligomerization, that FeCl3 increased significantly (p < 0.001) Tat oligomerization, and that DFO blocked significantly (p < 0.05) FeCl3-induced Tat oligomerization. B HIV-1 Tat from two sources (JHU and ABL) contained oligomerized Tat, but DFO was able to reverse the oligomerization when Tat protein (7.0 μg/ml) was incubated with DFO (100 and 500 μM) for 3 h at room temperature, and samples were separated using 4–16% Bis–Tris native gels (Thermo Fisher). C U87MG cells were incubated with HIV-1 Tat (2 μg/ml) and CQ (100 μM) for 4 h prior to 48 h incubation with FeCl3 (20 μM), DFO (20 μM), or FeCl3 (20 μM) plus DFO (20 μM). Tat-mediated HIV-1 LTR transactivation was decreased significantly (p < 0.05) by FeCl3, was increased significantly (p < 0.05) by DFO, and DFO blocked significantly (p < 0.05) FeCl3-induced decreases in Tat-mediated HIV-1 LTR transactivation. D U87MG cells were incubated with HIV-1 Tat protein (2 μg/ml) for 4 h in the presence of CQ (100 μM) prior to the addition of DFO (0.25 to 20 μM) and incubation for an additional 24 h. DFO significantly increased Tat-mediated HIV-1 LTR transactivation starting at 1.0 μM. (n = 3; *p < 0.05, **p < 0.01, ****p < 0.0001)
