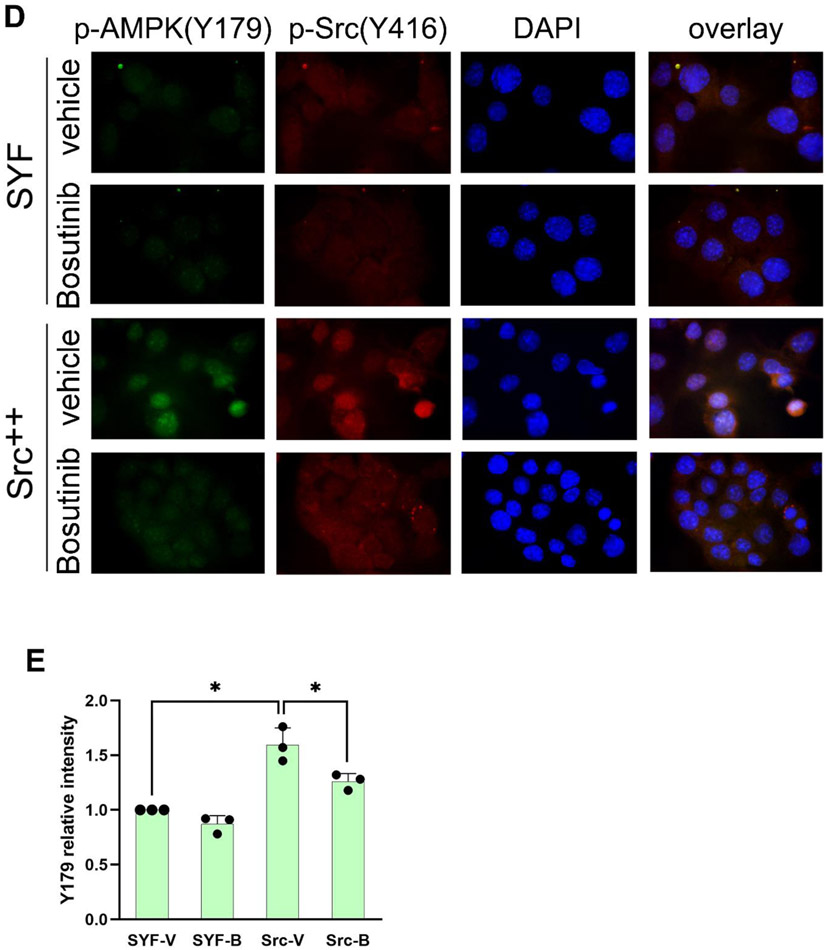
Figure 4. Src phosphorylates AMPK-α on Y179.
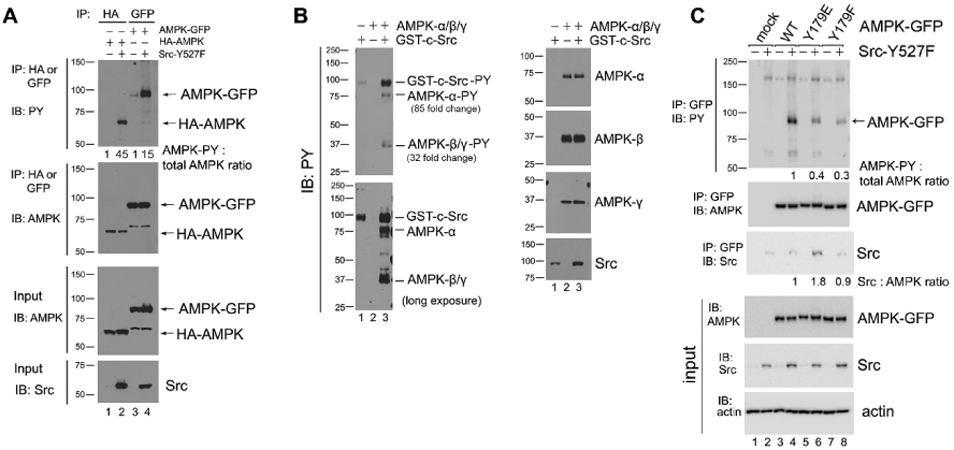
(A) AMPK-α subunit phosphorylation in Src-Y527F transfected cells. Immunoprecipitation with the indicated antibodies was performed in 293T cells that had been co-transfected with either HA-tagged or GFP-tagged AMPK-α2 along with Src-Y527F, and tyrosine-phosphorylated tagged AMPK-α2 was detected by immunoblot with HRP-conjugated anti-PY20 antibody. Quantification was performed as in previous figures by using cells transfected with HA-AMPK (lane 1) or AMPK-GFP (lane 3) in the absence of Src-Y527F as the normalized control, respectively. We surmise that the bands of 65 kD in molecular weight that are visible in anti-AMPK blots in AMPK-GFP lanes are degradation products of the AMPK-GFP protein. In all panels for Fig. 4, results shown are representative of at least three independent experiments with similar results. (B) In vitro phosphorylation of AMPK by Src. Recombinant AMPK-α/β/γ protein complex and GST-tagged Src kinase were incubated together in a kinase buffer and 200 μM ATP at 35°C for 1 hr. Tyrosine phosphorylation of AMPK was examined by immunoblotting using anti-PY20 antibody in the left panel, and total protein amounts were examined by immunoblotting with the indicated antibodies in the right panel. Quantification of tyrosine phosphorylation was performed by using AMPK in the absence of GST-c-Src (lane 2) as the normalized control. The loading amount of Src in lane 1 was 1/3 of that in lane 3. “Long exposure” indicates 10 times longer exposure compared to the top panel. (C) Tyrosine phosphorylation of the AMPK-Y179 mutants in Src-Y527F transfected cells. AMPK-α2-GFP was immunoprecipitated from the extracts of 293T cells that had been transfected or not (mock) with the various GFP-tagged AMPK-α2 constructs along with Src-Y527F as indicated, and tyrosine-phosphorylated AMPK-α2-GFP was detected by immunoblot with HRP-conjugated anti-PY20 antibody in the uppermost panel. Co-precipitation of Src in the various AMPK-Y179 immunoprecipitates was assessed in the third uppermost panel. Quantification in each case was performed by using cells expressing WT-AMPK-GFP in the presence of Src-Y527F (lane 4) as the normalized control. (D) Immunostaining with anti-p-AMPK(Y179) antibody. SYF and Src++ cells were cultured on coverslips, treated with or without 10 μM Bosutinib for 1 hr, and the phosphorylation of AMPK(Y179) and Src(Y416) sites was detected by immunofluorescence with the relevant phospho-specific antibodies. p-Src(Y416) indicates Src activation. DAPI-staining is also shown, along with the three color overlay. (E) Fluorescence intensity of p-AMPK(Y179) staining was determined using ImageJ software. SYF-V and SYF-B, vehicle- and Bosutinib-treated SYF cells, respectively; Src-V and Src-B, vehicle- and Bosutinib-treated Src++ cells, respectively. The statistical analyses were performed using Student's t-test with Graphpad Prism software. *p <0.05 (n = 3). For quantification and statistical analysis of the independent blots for Figs. 4A, B and C, please see Supplementary Figure 1.