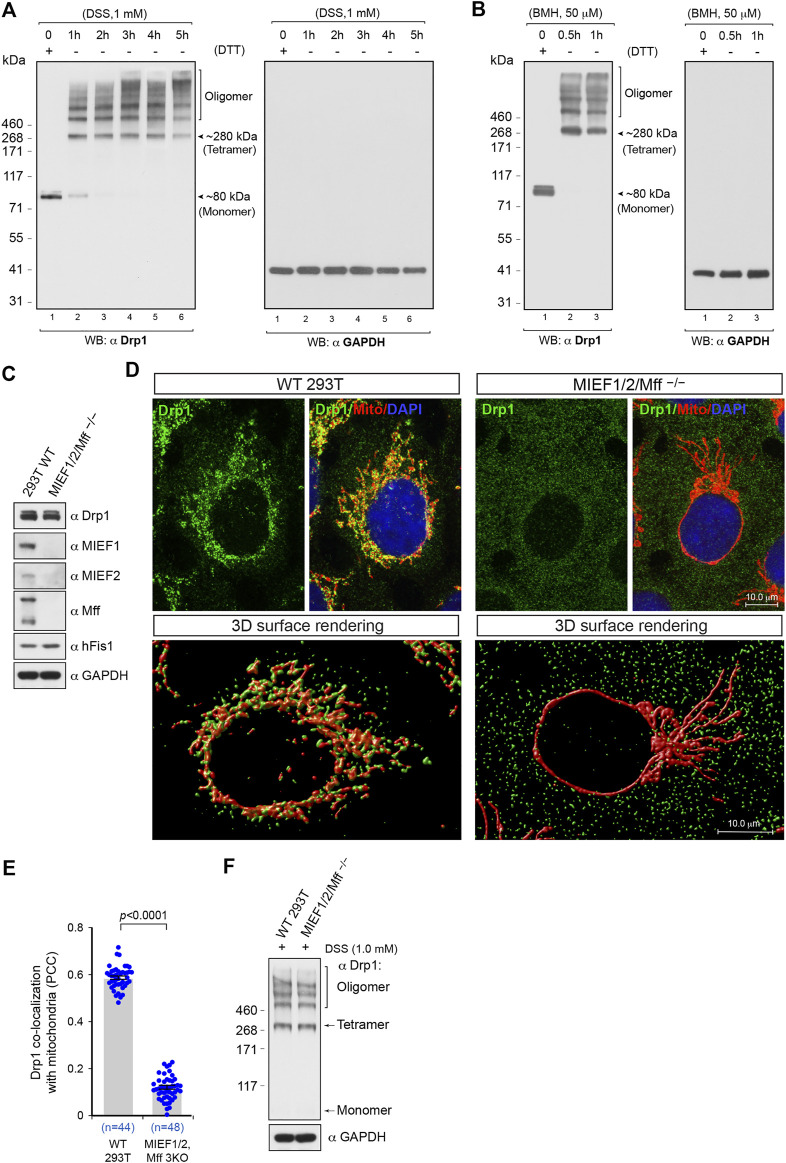
FIGURE 1.
The oligomeric state of Drp1 in human (293T) cells in vivo and the receptor-mediated recruitment of Drp1 to mitochondria enhances Drp1 oligomerization. (A,B) In 293T cells, Drp1 exists as multiple self-assembly subunits including the minimal self-assembly unit at ∼280 kDa (probably corresponding to tetramers) and several higher order units. 293T cells were treated with the cell-permeable crosslinking reagent DSS (A) or BMH (B) in a time course as indicated. Cell lysates were analyzed by Western blotting as indicated. The endogenous monomeric protein GAPDH was used as negative control to rule out nonspecific crosslinking. (C) The single cell derived colony of MIEF1/2/Mff−/− 293T cells (triple receptor knock-out) was validated by Western blotting analysis with indicated antibodies. WT 293T cells were used as control. (D) Confocal images and 3D surface rendering of mitochondrial morphology and endogenous Drp1 distribution in WT and MIEF1/2/Mff−/− 293T cells. Cells growing on glass coverslips were stained with MitoTracker (red) before fixation, followed by immunostaining with anti-Drp1 antibody (green). The cell nuclei were stained by DAPI (blue) (upper panels). 3D Surface rendering of confocal images with Drp1 (green) and mitochondria (red) in WT (lower left panel) and MIEF1/2/Mff−/− 293T cells (lower right panel). (E) Quantitative co-localization of endogenous Drp1 with mitochondria in (D) was analyzed by the PCC (mean ± S.E.M.). n represents the number of cells analyzed. (F) The oligomeric states of endogenous Drp1 in WT or MIEF1/2/Mff−/− 293T cells. Cultured cells were treated with freshly-prepared 1 mM DSS for 3 h at room temperature, followed by immunoblotting with indicated antibodies.