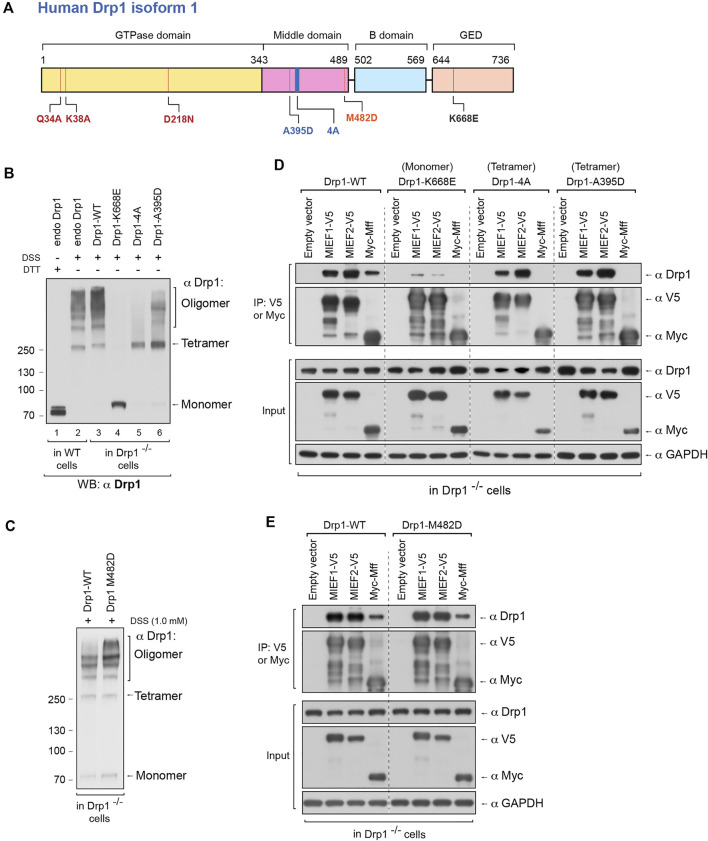
FIGURE 3.
The self-assembly state of Drp1 differentially regulates its binding to the mitochondrial receptors MIEFs and Mff. (A) Schematic representation of human Drp1 protein structure and functional domains. The positions of Drp1 mutations used in this study are indicated. (B) The oligomeric states of Drp1-WT, Drp1-K668E, Drp1-4A, and Drp1-A395D. Drp1−/− 293T cells were transfected with untagged wild-type Drp1 (Drp1-WT) or untagged Drp1 mutants as indicated for 18 h, treated with in vivo chemical crosslinker DSS (1 mM) for 3 h and analyzed by Western blotting with anti-Drp1 antibody. Endogenous Drp1 in wild-type 293T cells (endo Drp1) and exogenous Drp1-WT in Drp1−/− 293T cells were used as controls. (C) The oligomeric state of Drp1-M482D. Drp1−/− 293T cells were transfected with untagged Drp1-WT (control) or Drp1-M482D as indicated for 18 h and incubated with DSS (1 mM) for 3 h at room temperature, followed by Western blotting with anti-Drp1 antibody. (D) The Drp1 oligomerization-defective mutants Drp1-K668E, Drp1-4A, and Drp1-A395D interact differently with MIEFs and Mff. Drp1−/− 293T cells were co-transfected with indicated plasmids. Cell lysates were used for co-IP with anti-V5 or anti-Myc agarose beads as indicated, and the immunoprecipitates were analyzed by Western blotting with indicated antibodies. (E) The dominant oligomeric mutant Drp1-M482D shows intact binding with MIEFs and Mff. Drp1−/− 293T cells were co-transfected with indicated plasmids and subjected to co-IP. Cell lysates were incubated with anti-V5 or anti-Myc agarose beads as indicated. The proteins associated with MIEFs or Mff in immunoprecipitates were detected by Western blotting with indicated antibodies.