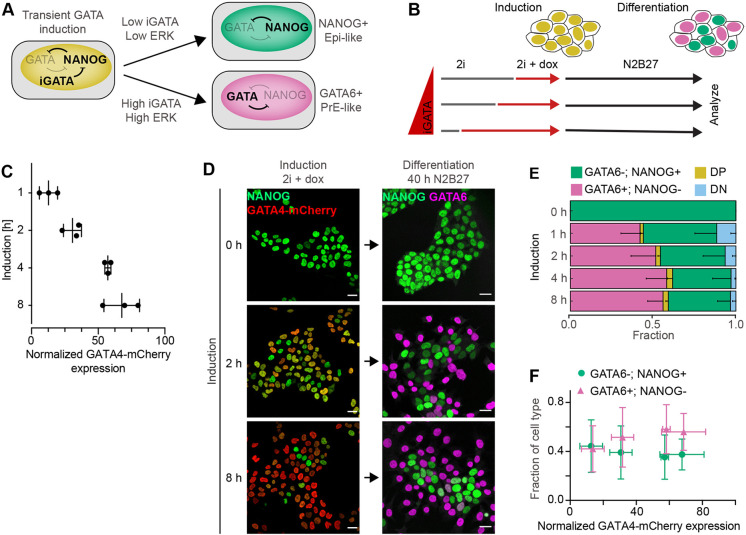
Fig. 1.
Proportions of differentiated cell types are independent from GATA4-mCherry induction levels. (A) Schematic of cell differentiation following transient doxycycline-controlled expression of inducible GATA factors (iGATA). (B) Experimental protocol for titrating inducible GATA4-mCherry expression levels by doxycycline addition to individual samples at different time points. The total time from seeding to analysis is held constant. (C) GATA4-mCherry expression levels for different durations of doxycycline induction in 2i+LIF medium measured by flow cytometry, normalized to the non-induced control. Individual data points show mean fluorescence intensities from at least 20,000 cells in an individual experiment, bars indicate mean±s.d. across n=3 independent experiments. (D) Left: immunostaining for NANOG (green) and GATA4-mCherry (red) in inducible cell lines immediately after the end of a doxycycline pulse of the indicated durations. Right: immunostaining for NANOG (green) and GATA6 (magenta) in cells treated with doxycycline for the indicated durations, followed by 40 h of differentiation in N2B27 medium. Cells without doxycycline induction had been continuously maintained in 2i medium. (E) Average cell-type proportions from n=4 independent experiments; the fraction of GATA6+; NANOG− cells is in magenta, GATA6−; NANOG+ cells is in green, double-positive cells (DP) is in yellow and double-negative cells (DN) is in blue. Error bars show 95% confidence intervals (CI). (F) Plot of average proportions of GATA6+; NANOG− cells (magenta) and GATA6−; NANOG+ cells (green) versus mean GATA4-mCherry levels for different doxycycline induction times. Individual datapoints correspond to different induction times, horizontal bars indicate ±s.d., vertical bars indicate 95% confidence interval. Scale bars: 20 µm.