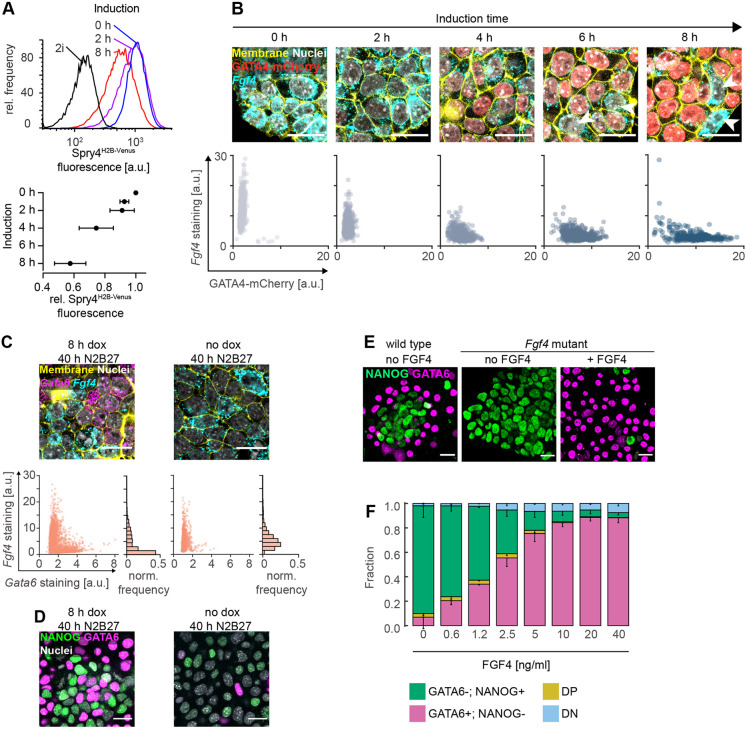
Fig. 2.
Differentiating ESCs communicate via FGF4. (A) Top: flow cytometry histograms showing Spry4H2B-Venus reporter expression after 24 h of differentiation in N2B27 medium following the indicated durations of doxycycline induction. Black line indicates reporter expression in cells maintained in 2i medium. Bottom: Mean±s.d. of reporter expression from n=4 independent experiments, normalized to fluorescence levels of cells transferred to N2B27 without doxycycline induction. (B) Top: GATA4-mCherry protein (red) and Fgf4 mRNA expression (cyan) in inducible cells at indicated durations of doxycycline induction. Bottom: corresponding single cell quantifications. (C) Top: Gata6 (magenta) and Fgf4 mRNA (cyan) expression in inducible cells after 40 h of culture in N2B27 following an 8 h doxycycline pulse (left) or following transfer to N2B27 without induction (right). Bottom: corresponding single cell quantifications. Cell membranes and nuclei in B,C labeled with CellBrite (yellow) and Hoechst 33342 (white), respectively. (D) Immunostaining of cells treated as in C for GATA6 (magenta) and NANOG (green). Nuclei stained with Hoechst 33342 (white). (E) Immunostaining for GATA6 (magenta) and NANOG (green) in wild-type (left) and Fgf4-mutant cells differentiated for 40 h in N2B27 without (middle) or with (right) 10 ng/ml FGF4 after an 8 h doxycycline pulse. (F) Average proportions of cell types in Fgf4-mutant cells induced with doxycycline for 8 h, followed by differentiation in N2B27 in the presence of the indicated concentrations of FGF4. GATA6 and NANOG expression were detected by immunostaining and measured by flow cytometry (see Fig. S5). n=4; the fraction of GATA6+; NANOG− cells is in magenta, GATA6−; NANOG+ cells is in green, double-positive cells (DP) is in yellow, and double-negative cells (DN) is in blue. Error bars are 95% confidence intervals. Scale bars: 20 µm in B-E.