Abstract
Background
Strategies for managing respiratory motion, specifically motion-encompassing methods, in radiation therapy typically assume reproducible breathing. In reality, respiratory motion variations occur and ultimately cause tumor motion variations, which can result in differences between the planned and delivered dose distributions. Therefore, breathing guidance techniques have been investigated to improve respiratory reproducibility. To our knowledge, bilevel positive airway pressure (BIPAP) ventilation assistance has not been previously investigated as a technique for improving respiratory reproducibility and is the focus of this work.
Methods and Materials
Ten patients undergoing radiation therapy treatment for cancers affected by respiratory motion (eg, lung and esophagus) participated in sessions in which their breathing was recorded during their course of treatment; these sessions occurred either before or after radiation treatments. Both unassisted free-breathing (FB) and BIPAP ventilation-assisted respiratory volume data were collected from each patient using spirometry. Patients used 2 different BIPAP ventilators (fixed BIPAP and flexible BIPAP), each configured to deliver the same volume of air per breath (ie, tidal volume). The flexible BIPAP ventilator permitted patient triggering (ie, it permitted patients to initiate each breath), and the fixed BIPAP did not. Intrasession and intersession metrics quantifying tidal volume variations were calculated and compared between the specific breathing platforms (FB or BIPAP). In addition, patient tolerance of both BIPAP ventilators was qualitatively assessed through verbal feedback.
Results
Both BIPAP ventilators were tolerated by patients, although the fixed BIPAP was not as well tolerated as the flexible BIPAP. Both BIPAP ventilators showed significant reductions (P < .05) in intrasession tidal volume variation compared with FB. However, only the fixed BIPAP significantly reduced the intersession tidal volume variation compared with FB.
Conclusions
Based on the established correlation between tidal volume and tumor motion, any reduction of the tidal volume variation could result in reduced tumor motion variation. Fixed BIPAP ventilation was found to be tolerated by patients and was shown to significantly reduce intrasession and intersession tidal volume variations compared with FB. Therefore, future investigation into the potential of fixed BIPAP ventilation is warranted to define the possible clinical benefits.
Introduction
Respiratory motion affects all tumor sites in the thorax and abdomen and can introduce localization uncertainties that can negatively affect the image acquisition, treatment planning, and radiation delivery of a patient's radiation treatment. To reduce the effect of respiratory variations in radiation therapy, motion management strategies have been developed, such as those described in the American Association of Physicists in Medicine Task Group 76 (AAPM TG-76) report on respiratory motion management.1 Strategies using free-breathing (eg, motion-encompassing methods) generally assume patients will reproduce the same respiratory pattern during image acquisition and each subsequent radiation delivery treatment. In reality, patients’ natural free-breathing patterns can vary from breath to breath (intrafraction) and day to day (interfraction).2, 3, 4 Both intrafraction and interfraction variations in the respiratory pattern cause tumor motion variations, which can result in differences between the planned and delivered dose distributions.1 Thus, breathing feedback and guidance techniques—ranging from simple audio buzzers to interactive guiding interfaces of the respiration signal—have been developed to improve patients’ respiratory reproducibility (ie, reduce respiratory pattern variations) with the goal of improving image quality and the accuracy of radiation delivery.2,5
To this end, continuous positive airway pressure (CPAP) ventilation has recently been investigated as a potential respiratory motion management technique in radiation therapy. CPAP ventilation is a form of noninvasive ventilation that delivers a constant stream of pressurized air to the upper airways and lungs throughout the respiratory cycle. Typically used to treat respiratory complications such as obstructive sleep apnea, acute respiratory failure, and chronic obstructive pulmonary disease, CPAP ventilation increases the baseline lung volume and has been shown to reduce total pulmonary power during inspiration (ie, making it easier for patients to breathe) compared with unassisted free-breathing.6,7 As for its potential as a respiratory motion management technique in radiation therapy, Goldstein et al found that CPAP ventilation increased total lung volume, reduced tumor motion, and reduced lung and heart dose compared with free-breathing for a cohort of 10 patients being treated with stereotactic body radiation therapy.8 Although Di Perri et al also observed that CPAP ventilation increased lung volume, they found it had negligible effect on tumor motion and led to a small decrease in lung dose for 20 patients undergoing stereotactic ablative radiation therapy.9 Anecdotally, CPAP ventilation has been shown to reduce the heart and lung dose in patients with respiratory complications, namely deep inspiration breath holds.10, 11, 12 Although these results are mixed, they are generally encouraging for using CPAP ventilation as a technique to reduce tumor motion and dose to healthy tissues. However, the mechanism for these reductions is incidental to the imposed increase in total lung volume rather than direct control over the respiratory cycle. Therefore, the ability to consistently and robustly improve patients’ respiratory reproducibility may not be accomplished with CPAP ventilation alone.
Bilevel positive airway pressure (BIPAP) ventilation is another noninvasive ventilation technique that delivers alternating high and low pressures during inhalation and exhalation phases of the breathing cycle, as opposed to CPAP ventilation's single, constant pressure. Although BIPAP ventilation and CPAP ventilation are used for similar respiratory complications, the difference in positive pressures during inhalation and exhalation using BIPAP ventilation provides assistance during patients’ breathing efforts, compared with both free-breathing and CPAP ventilation.13 Most commercially available BIPAP ventilators offer volume-targeted modes, which aim to assist patients’ breathing efforts by delivering the same tidal volume (ie, the volume of air inhaled and exhaled) with each breath. Based on the demonstrated strong positive correlation between tidal volume and tumor motion, improving patients’ respiratory reproducibility could ultimately improve tumor motion reproducibility.14
Given that most breathing guidance techniques, including CPAP, do not assist or augment patients’ breathing to achieve consistent (ie, reproducible) tidal volume respiratory patterns, exploring the utility of BIPAP ventilation in radiation therapy is a logical progression. BIPAP ventilation assistance is designed to assist the user's breathing and can be configured to deliver the same volume of air with each breath (eg, volume-targeted modes), which may result in advantages when using respiratory-induced tumor motion management methods such as motion-encompassing, as discussed in the AAPM TG-76 report. To our knowledge, BIPAP ventilation has not been investigated as a technique to improve respiratory reproducibility in patients receiving radiation therapy. We hypothesized that BIPAP ventilation would reduce both intrafractional and interfractional variations in tidal volume compared with free-breathing. Therefore, the purpose of this work was to investigate the feasibility of using BIPAP ventilation in radiation oncology by (1) assessing whether patients with cancer can tolerate BIPAP ventilation assistance and (2) evaluating if BIPAP ventilation assistance improves respiratory reproducibility in terms of consistency in tidal volume and breathing period. The results of this pilot study are important for determining whether future studies are warranted in exploring BIPAP ventilation's potential for improving targeting accuracy, treatment plan quality, and delivery accuracy of radiation therapy.
Methods and Materials
We obtained institutional review board approval to collect breathing data from patients with cancer who were undergoing radiation therapy. Daily breathing sessions, which were independent from treatments, occurred in an open examination room either before or after patient treatments, depending on the patients’ schedules, and involved breathing with and without BIPAP ventilation. Candidates for study enrollment were adult patients who met the following criteria: they had disease sites affected by respiratory motion; they were to be treated with normal breathing as prescribed by a physician because BIPAP ventilation is intended to assist normal breathing treatments; they were able to tolerate a nasal ventilation mask and breathe through their nose; and they were amenable to coaching for their breathing. Written informed consent was obtained for all participants meeting these criteria on their initial imaging simulation day.
We used 2 different commercially available BIPAP ventilators in this study: the Philips Respironics V60 BIPAP ventilator (Philips Respironics California, LLC, Carlsbad, California) and the Lifecare Personal Lightweight Ventilator (PLV) 100 BIPAP (Respironics, Inc, Murrysville, Pennsylvania), referred to here as BIPAP 1 and BIPAP 2, respectively. BIPAP 1 is a microprocessor-controlled, pneumatic blower ventilator equipped with a volume-targeted mode called the average volume-assured pressure support mode, which aims to maintain a target tidal volume during each breath by monitoring previous tidal volumes and continuously adjusting the delivered pressures. Patient triggering is permitted by BIPAP 1, allowing patients to initiate (ie, trigger) each breath and to also control their tidal volume (ie, how much air is inhaled and exhaled with each breath). BIPAP 2 is a microprocessor-controlled, piston-driven ventilator with a volume-targeted mode called the control mode. The control mode delivers all breaths at a preset tidal volume and breathing period, therefore prohibiting patient triggering.
Ten patients were enrolled in this study and were fitted with a nasal ventilation mask and bacteria filter. The mean participant age was 58 years (range, 34-75 years) (Table 1). A smoking history was obtained from all patients, which ranged from an unknown number of pack-years to 90 pack-years (1 pack-year = 7300 cigarettes). All patients participated in sessions on more than 50% of their treatment days. Not all patients had the same number of sessions, because patients were prescribed different numbers of radiation treatment fractions. Patients were also permitted to skip sessions on treatment days when they were not feeling well, running late, or had other appointments (eg, undertreatment physician visits, chemotherapy, etc).
Table 1.
Patient characteristics
| Patient | Age, y | Gender | Treatment site | Smoking history, pack-years* | Treatment fractions | Sessions |
|---|---|---|---|---|---|---|
| 1 | 57 | M | Esophagus | 31-90 | 28 | 25 |
| 2 | 61 | M | RLL | 31-90 | 30 | 21 |
| 3 | 75 | M | RUL | <30 | 33 | 17 |
| 4 | 57 | M | Esophagus | <30 | 28 | 16 |
| 5 | 59 | F | RLL | 31-90 | 33 | 17 |
| 6 | 66 | M | LL | U | 30 | 18 |
| 7 | 43 | F | RUL | <30 | 33 | 17 |
| 8 | 71 | M | Esophagus | <30 | 28 | 15 |
| 9 | 34 | M | LLL | <30 | 4 | 4 |
| 10 | 52 | F | LL | <30 | 7 | 4 |
Abbreviations: LL = left lung; LLL = left lower lung; RLL = right lower lung; RUL = right upper lung; U = unknown.
1 pack-year = (1 pack/d) × (20 cigarettes/pack) × (365 days/y) × (1 year) = 7300 cigarettes.
Patients participated in sessions lasting approximately 10 minutes each, during which breathing data for each platform (free-breathing [FB], BIPAP 1, and BIPAP 2) was collected for 2 minutes—approximating the beam-on time of a typical volumetric modulated arc therapy (VMAT) beam. Patients wore their specific nasal ventilation mask and were then immobilized using the same devices used for their radiation treatments. FB data were always collected at the beginning of each session. After the completion of the FB data collection, patients were connected to either BIPAP 1 or BIPAP 2, with the order alternating with each session to mitigate any potential bias.
Patient-specific BIPAP ventilator settings (eg, tidal volumes and breathing periods) were determined using verbal patient feedback during the first session and were used for subsequent sessions. After the FB data collection during the first session, patients were given an overview of the BIPAP ventilators and their functionality. Patients were then connected to a ventilator and instructed to breathe normally. Patient-specific ventilator settings were tuned using verbal feedback until each patient was comfortable with the ventilator settings of the given BIPAP. This selection of ventilator settings lasted approximately 5 to 10 minutes before BIPAP breathing data were collected. This process was then repeated for the remaining BIPAP ventilator. During subsequent sessions, after the FB data collection was complete, patients had a short warm-up period (10-90 seconds) using the BIPAP ventilator before the BIPAP data collection began.
Spirometry was used to measure respiratory-volume data. A mass flow sensor was coupled to the nasal mask and used to measure FB and BIPAP 2 breathing data. Patients were also visually monitored during sessions to ensure their mouth remained closed and all breathing was only through the nasal mask. Because BIPAP 1 continuously adjusted the delivered pressures (ie, the delivered baseline volume was not constant), the mass flow sensor could not be used to measure the respiratory-volume data. Instead, breathing data for BIPAP 1 were extracted using in-house signal processing software. The anterior abdominal surface was also monitored using an in-house abdominal surface marker system designed to provide consistent measurements of breathing-pattern periods across the FB, BIPAP 1, and BIPAP 2 breathing platforms.
Patients were provided visual feedback of their real-time volume waveform when using BIPAP 1 because it permitted patient triggering, although they were not required to actively watch this feedback. A projector, which displayed the real-time volume waveform from the device's display screen, was mounted to the patient couch and aimed at the ceiling directly above the patient's head. A dotted line at the target tidal volume level was superimposed on the volume waveform to help guide the patients, as shown in Figure 1. Because BIPAP 2 did not permit patient triggering, visual cues timed to inhalation and exhalation were provided when using BIPAP 2, although patients were not required to actively watch them. An in-house visual cue stand, which contained a green “inhale” light-emitting diode (LED) and a red “exhale” LED, was clamped to the couch and hung above the patient's head, as shown in Figure 2. Electronic timing from BIPAP 2 was used to synchronize the inhale and exhale LEDs with the air volume output of the ventilator. Patients were instructed to inhale when the green LED was illuminated and to exhale when the red LED was illuminated.
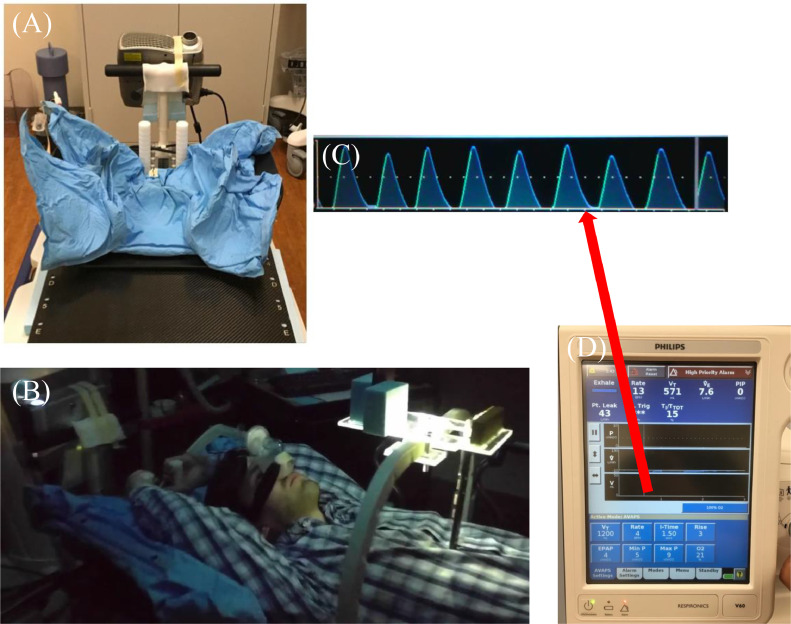
Fig. 1.
Overview of BIPAP 1 (Philips Respironics V60). (A) Patient immobilization and projector pointed at ceiling. (B) Model wearing nasal mask and bacteria filter with abdominal surface marker in place. (C) Example patient respiratory volume waveform provided by device display screen. (D) Device display screen which displayed the real-time respiratory volume waveform.
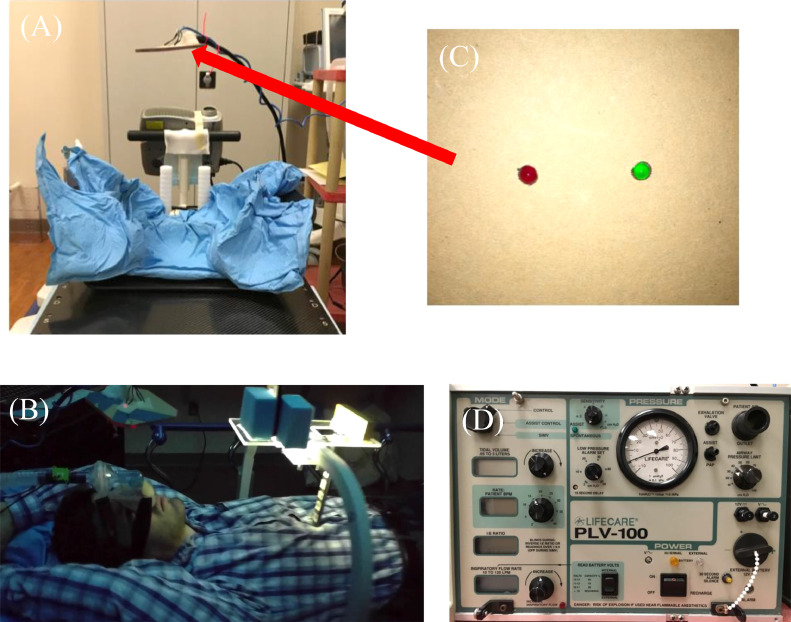
Fig. 2.
Overview of BIPAP 2 (Lifecare PLV-100). (A) Patient immobilization and hanging visual cue stand. (B) Model wearing nasal mask, bacteria filter, and mass flow sensor with abdominal surface marker in place. (C) Inhale and exhale visual cues on hanging visual cue stand. (D) Front face of BIPAP 2.
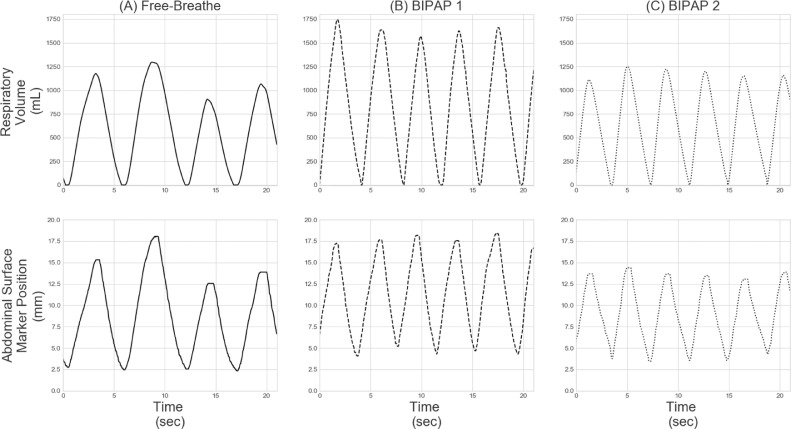
A sample of the breathing data collected during a patient's session, specifically the tidal volumes and abdominal surface marker peak-to-peak periods, is shown in Figure 3. For each session, the coefficient of variation (CV) was calculated from the mean and standard deviation of the tidal volumes measured with each platform (FB, BIPAP 1, or BIPAP 2). Intrasession variation was defined as the mean of the CVs of all sessions for each platform. In addition, for each session, the mean tidal volume was normalized to the first session's tidal volume mean. Intersession variation was defined as the standard deviation of the relative session means for each platform. This analysis was repeated for the abdominal peak-to-peak periods for each platform. Intrasession and intersession variations were calculated for each patient. Results of the tidal volume and abdominal surface marker peak-to-peak period variations for all patients were compared between platforms (FB vs BIPAP 1, FB vs BIPAP 2, and BIPAP 1 vs BIPAP 2). In addition, patient tolerance of BIPAP ventilation was qualitatively assessed by verbal feedback, and the mean data collection times were calculated and compared. Statistical significance was determined for each comparison using the nonparametric Wilcoxon signed rank test, with the significance level set at .05.
Fig. 3.
Patient example of the breathing data collected during a session. TOP ROW: Respiratory volume for (A) Free-breathe, (B) BIPAP 1, and (C) BIPAP 2. BOTTOM ROW: Abdominal surface marker position for (A) Free-breathe, (B) BIPAP 1, and (C) BIPAP 2. Components of the breathing data that were analyzed included the tidal volumes (i.e. volume of air exhaled each breath) and abdominal surface marker periods.
Results
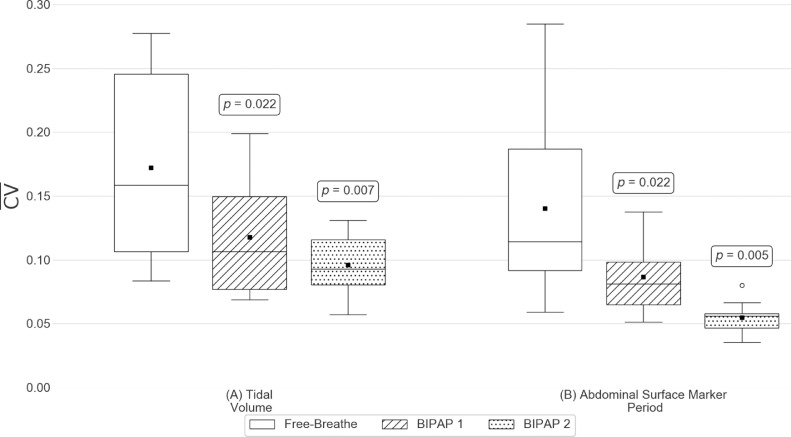
A summary of the intrasession and intersession variation results is shown in Figures 4 and 5, respectively. The mean and standard deviation of the intrasession tidal volumes and abdominal surface marker periods are shown for all patients in Table 2.
Fig. 4.
Intrasession variation results for (A) Tidal volume and (B) Abdominal surface marker period. Patient distributions of the intra-session coefficient of variations (CV) of each metric for each breathing platform. Here, black squares indicate the distribution means, black horizontal lines indicate the distribution medians, and white circles indicate suspected outliers (i.e. data points lying outside 1.5 times the interquartile range). p-values shown are results from statistical tests between the free-breathe and the associated BIPAP platform.
Fig. 5.
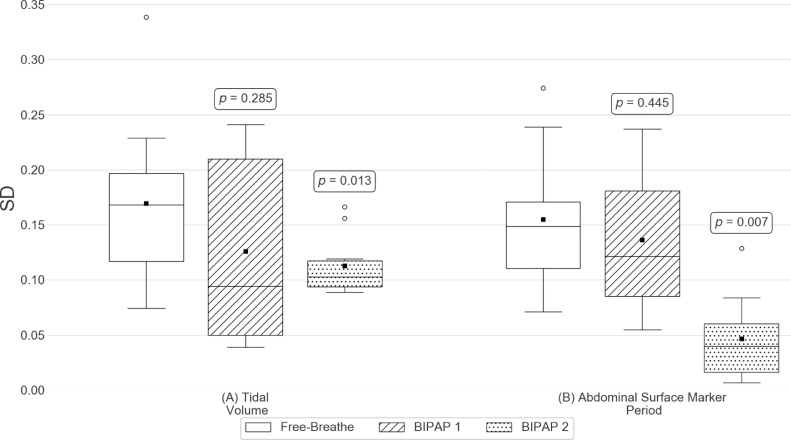
Intersession variation results for (A) Tidal volume and (B) Abdominal surface marker period. Patient distributions of the intersession standard deviation (SD) of each metric for each breathing platform. Here, black squares indicate the distribution means, black horizontal lines indicate the distribution medians, and white circles indicate suspected outliers (i.e. data points lying outside 1.5 times the interquartile range). p-values shown are results from statistical tests between the free-breathe and the associated BIPAP platform.
Table 2.
Summary of the intrasession tidal volumes and abdominal surface marker periods for all patients
| Tidal volume |
Abdominal surface marker period |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FB | BIPAP 1 | BIPAP 2 | FB | BIPAP 1 | BIPAP 2 | |||||||
| Patient | Mean (s) | SD (s) | Mean (s) | SD (s) | Mean (s) | SD (s) | Mean (s) | SD (s) | Mean (s) | SD (s) | Mean (s) | SD (s) |
| 1 | 658.6 | 172.2 | 797 | 144.7 | 653.8 | 85.7 | 3.7 | 0.8 | 3.2 | 0.4 | 3.9 | 0.3 |
| 2 | 737.8 | 197.7 | 1417.8 | 161.3 | 903.4 | 83.5 | 4.6 | 1.3 | 3.8 | 0.3 | 3.8 | 0.2 |
| 3 | 804.6 | 85.8 | 1912.6 | 162.3 | 1100 | 89.6 | 4.8 | 0.5 | 5.8 | 0.4 | 4.7 | 0.2 |
| 4 | 444.3 | 63.6 | 472.2 | 59.5 | 452.6 | 55.6 | 2.5 | 0.3 | 1.7 | 0.1 | 2.5 | 0.1 |
| 5 | 441.8 | 87.9 | 613.3 | 96.5 | 595.2 | 56.4 | 2.8 | 0.4 | 2.2 | 0.3 | 3 | 0.2 |
| 6 | 554.5 | 46.4 | 703.6 | 69.9 | 575.8 | 53.7 | 3 | 0.2 | 2.9 | 0.2 | 3 | 0.2 |
| 7 | 1006 | 104.2 | 1379.8 | 97.9 | 988.3 | 56.2 | 6.8 | 0.6 | 7.9 | 0.8 | 7.3 | 0.4 |
| 8 | 864.2 | 92.2 | 1040.8 | 71.4 | 963.8 | 75.3 | 6.2 | 0.5 | 5.8 | 0.3 | 5.5 | 0.2 |
| 9 | 1262.7 | 350.4 | 1445.1 | 107.1 | 965.3 | 77.3 | 12 | 2.5 | 6.5 | 0.5 | 6.7 | 0.3 |
| 10 | 425.2 | 73.9 | 449.6 | 89.5 | 654.5 | 83.1 | 3.9 | 0.5 | 3.7 | 0.3 | 4.4 | 0.2 |
Abbreviations: BIPAP = bilevel positive airway pressure; FB = free-breathing; SD = standard deviation.
The mean intrasession tidal volume variations of all patients were 0.172 for FB, 0.118 for BIPAP 1, and 0.096 for BIPAP 2. The intrasession tidal volume variation of both BIPAP 1 (P = .02) and BIPAP 2 (P = .007) was significantly lower than that of FB. BIPAP 2 showed significantly lower intrasession tidal volume variation compared with BIPAP 1 (P = .047). The mean intersession tidal volume variations of all patients were 0.169 for FB, 0.126 for BIPAP 1, and 0.113 for BIPAP 2. Only the intersession tidal volume variations of BIPAP 2 were significantly different compared with those of FB. There also was no significant difference in intersession tidal volume variation between BIPAP 1 and BIPAP 2.
The mean intrasession abdominal surface marker period variations of all patients were 0.140 for FB, 0.086 for BIPAP 1, and 0.055 for BIPAP 2. Intrasession abdominal surface marker period variations of both BIPAP 1 (P = .02) and BIPAP 2 (P = .005) were significantly lower than those of FB. The intrasession abdominal surface marker period variation of BIPAP 2 was significantly lower than that of BIPAP 1 (P = .007). The mean intersession abdominal surface marker period variations of all patients were 0.155 for FB, 0.136 for BIPAP 1, and 0.046 for BIPAP 2. There was no significant difference in intersession abdominal surface marker period variation between BIPAP 1 and FB, whereas BIPAP 2 showed significantly lower intersession abdominal surface marker period variations compared with FB (P = .007). In addition, the intersession abdominal surface marker period variation of BIPAP 2 was significantly lower than that of BIPAP 1 (P = .005).
BIPAP 1 was well tolerated, and none of the patients mentioned any discomfort. The mean (SD) BIPAP 1 data collection time among all patients (first abdominal surface marker peak time to last abdominal surface marker peak time, 110.5 [6.8] seconds) was not significantly different than for FB (112.0 [5.0] seconds). On the other hand, BIPAP 2 was not as well tolerated. Four patients reported difficulty using BIPAP 2, especially breathing at the fixed period, and mentioned that it felt restrictive or that their breaths were “cut off.” The mean (SD) BIPAP 2 data collection time of all patients (75.8 [24.3] seconds) was significantly less than for FB (112.0 [5.0] seconds) (P = .005).
Discussion
To our knowledge, this is the first study to evaluate the feasibility of using BIPAP ventilation to help patients undergoing radiation therapy improve their respiratory reproducibility. We hypothesized that compared with free-breathing, using BIPAP ventilation would result in reduced tidal volume variation both breath to breath (intrasession) and day to day (intersession). BIPAP ventilation was tolerated well by patients and significantly reduced both intrasession and intersession mean tidal volume variations, as well as breathing-period variations, compared with unassisted free-breathing.
Based on the established positive correlation between respiratory volume (ie, tidal volume) and tumor motion,14 we assumed tidal volume was an acceptable surrogate for tumor motion. We also used an abdominal surface marker to provide a monitoring system that was consistent across both the FB and BIPAP data collection platforms. The abdominal surface marker periods were used to provide temporal information about the respiration pattern.
Patients’ FB tidal volume respiratory patterns had both intrasession and intersession variations. Dosimetrically, breath-to-breath and day-to-day tumor motion variations could result in decreased tumor coverage and, consequently, increased dose to surrounding normal tissues. Tumor motion variations can be accounted for by using larger treatment margins to ensure complete coverage of the prescription dose1,15; however, owing to normal-tissue tolerances, this approach may not be applicable in hypofractionated treatment regimens such as stereotactic body radiation therapy techniques. Given these potentially catastrophic consequences of tumor motion variations, reducing respiratory tidal volume variations is crucial. In this study, BIPAP ventilation significantly reduced intrasession tidal volume variations. This finding may warrant future studies into how these reductions translate to improvements both in targeting accuracy via increased image quality and in radiation treatment delivery. However, it is possible to estimate reductions in tumor motion variation with the results of the present study by assuming a mean tumor motion, as reported by Hoisak et al, of 2.5 cm and a one-to-one correlation between tidal volume and tumor motion.1,14 This suggests that a tidal volume CV of 0.09, the median CV found for BIPAP 2, results in 2.3 mm of tumor motion variation (ie, standard deviation). Similarly, a tidal volume CV of 0.16, the median CV found for FB, results in 4 mm of tumor motion variation. This approximately 2-mm improvement in tumor motion reproducibility using BIPAP ventilation could lead to more reproducible motion during radiation delivery or even a reduction of the margins added when using the motion-encompassing methods, which currently are 2 to 5 mm. This study's results also showed that BIPAP ventilation significantly reduced intersession tidal volume variations. In other words, patients using BIPAP ventilation reproduced their first-session tidal volumes significantly better during subsequent sessions, compared with free-breathing. The effect of reduced interfraction tidal volume variation on tumor motion variation still needs to be investigated in an imaging and dosimetric study.
Analogous to this study's observations of tidal volume respiratory patterns, patients’ FB abdominal surface marker periods showed both intrasession and intersession variations. We found that BIPAP ventilation assistance significantly reduced both intrasession and intersession variations in abdominal surface marker periods compared with FB. Neicu et al suggested that a reproducible abdominal surface marker period is required to predict tumor position and synchronize the radiation field with the tumor motion.16,17 The significant reductions in intrasession and intersession abdominal surface marker period variations with BIPAP ventilation assistance could lead to improvements in tumor position predictions or in respiratory gating techniques in which reproducible tumor motion periods are desirable.
This study had limitations beyond using a small sample size of 10 patients. Although all patients had sessions on more than 50% of their treatment days, they were permitted to skip sessions when they were not feeling well, were running late, or had other appointments. Subsequently, the overall effecy of using ventilation assistance was not fully investigated, which would have required having sessions on all treatment days. Also, 7 patients had mean BIPAP 2 data collection times shorter than 90 seconds. This was likely a consequence of BIPAP 2’s control mode that prevents patient-triggering; most patients were unable to use BIPAP 2 for sustained periods before having to open their mouth to “catch their breath.” This led to shorter data collection times for these patients, because BIPAP 2 data collection during the initial session—which determined the length of subsequent sessions—was terminated if patients either had to open their mouth to catch their breath or if they notified the investigator that they could not use BIPAP 2 any longer. Among the patients who had difficulty using BIPAP 2 for more than 2 minutes, there was a range in age, smoking history, and overall comfort in using the device. In addition, although breathing data were collected during 2-minute intervals to simulate the beam-on time of a typical VMAT arc, typical treatment sessions can last up to and beyond 30 minutes owing to patient setup, imaging, and multiple beams. Based on feedback from patients in this study and the data-collection-time results for BIPAP 2, requiring patients to use BIPAP ventilation assistance without patient triggering throughout the entire treatment session is most likely not feasible without additional considerations. Since this study was conducted, a relief valve has been inserted into the BIPAP patient circuit to enable free breathing during times when BIPAP use is not required (eg, between patient setup and daily imaging). Anecdotally, users of BIPAP 2 with the relief valve inserted have expressed an easier time using the device.
The results of this study, which suggest that both intrasession and intersession patient respiratory reproducibility may be improved using BIPAP ventilation, warrant further investigation into the possible clinical benefits of this respiratory management technique in radiation oncology. The primary advantage of BIPAP ventilation compared with other patient monitoring systems such as surface-guided radiation therapy systems, which typically use a passive patient monitoring and positioning system, is that BIPAP ventilation is an active system that assists the patient's breathing directly to achieve consistent or reproducible tidal volume respiratory patterns. Also, in this study, BIPAP ventilation required less than 1.5 minutes of patient warmup time, after the initial use and selection of patient-specific ventilator settings, to achieve more reproducible respiratory patterns compared with free-breathing. This warmup time is comparable to established CPAP ventilation systems. Further investigations are needed to evaluate the effect of using BIPAP ventilation on tumor motion variations. In this study, BIPAP 2 yielded the largest significant reductions in tidal volume and abdominal surface marker period variations; however, it was not as well tolerated for 2 minutes as BIPAP 1. We attribute this to a reduced warm-up time compared with that in a study by Goldstein et al8 and to the delivery characteristics of BIPAP 2—specifically, not permitting patient triggering. Because tidal volume is strongly correlated with tumor motion, a clinical imaging and dosimetric investigation is needed to assess the reduction in tumor motion variations using BIPAP ventilation. This would help elucidate the clinical benefits of using BIPAP ventilation assistance during radiation treatments. Despite the challenges of using BIPAP 2 for some patients, we recommend performing the clinical imaging and dosimetric investigation using BIPAP 2, with a relief valve inserted, based on its superior results for tidal volume and abdominal surface marker period variation compared with free-breathing and BIPAP 1.
Conclusions
Because modern strategies for managing respiratory motion in radiation therapy, specifically motion-encompassing methods discussed by AAPM TG-76, assume reproducible breathing, any variations in observed respiratory pattern magnitude and frequency can result in a suboptimal delivery of the prescribed dose. To improve patient respiratory reproducibility of patients undergoing radiation therapy, this study evaluated the use of BIPAP ventilation with 2 commercially available ventilators with different types of delivery settings. Compared with unassisted free-breathing, BIPAP ventilation was found to be tolerable and to significantly reduce variations in tidal volume and the period of an external surface marker. To our knowledge, this pilot study was the first to investigate and confirm the feasibility of BIPAP ventilation as a respiratory management technique in radiation therapy. Future work is warranted to further define the potential clinical advantages of using BIPAP ventilation to improve respiratory reproducibility, especially for institutions without existing motion management or surface-guided radiation therapy systems.
Footnotes
Sources of support: This work was partially supported by the Farve Family Innovation Award from the Mary Bird Perkins Cancer Center, Baton Rouge, Louisiana.
Disclosures: none.
References
- 1.Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76a. Med Phys. 2006;33:3874–3900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- 2.Kini VR, Vedam SS, Keall PJ, Patil S, Chen C, Mohan R. Patient training in respiratory-gated radiotherapy. Med Dosim. 2003;28:7–11. doi: 10.1016/S0958-3947(02)00136-X. [DOI] [PubMed] [Google Scholar]
- 3.Ge J, Santanam L, Noel C, Parikh PJ, et al. Planning 4-dimensional computed tomography (4dct) cannot adequately represent daily intrafractional motion of abdominal tumors. Int J Radiat Oncol Biol Phys. 2012;85:999–1005. doi: 10.1016/j.ijrobp.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Worm ES, Høyer M, Fledelius W, Hansen AT, Poulsen PR. Variations in magnitude and directionality of respiratory target motion throughout full treatment courses of stereotactic body radiotherapy for tumors in the liver. Acta Oncol. 2013;52:1437–1444. doi: 10.3109/0284186X.2013.813638. [DOI] [PubMed] [Google Scholar]
- 5.Pollock S, Keall R, Keall P. Breathing guidance in radiation oncology and radiology: A systematic review of patient and healthy volunteer studies. Med Phys. 2015;42:5490–5509. doi: 10.1118/1.4928488. [DOI] [PubMed] [Google Scholar]
- 6.Katz JA, Marks JD. Inspiratory work with and without continuous positive airway pressure in patients with acute respiratory failure. Anesthesiology. 1985;63:598–607. doi: 10.1097/00000542-198512000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Simon S, Collop N. Latest advances in sleep medicine: Obstructive sleep apnea. Chest. 2012;142:1645–1651. doi: 10.1378/chest.12-2391. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein JD, Lawrence YR, Appel S, et al. Continuous positive airway pressure for motion management in stereotactic body radiation therapy to the lung: A controlled pilot study. Int J Radiat Oncol Biol Phys. 2015;93:391–399. doi: 10.1016/j.ijrobp.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Di Perri D, Colot A, Delor A, et al. Effect of continuous positive airway pressure administration during lung stereotactic ablative radiotherapy: A comparative planning study. Strahlenther Onkol. 2018;194:591–599. doi: 10.1007/s00066-018-1278-2. [DOI] [PubMed] [Google Scholar]
- 10.Kil WJ. Novel use of continuous positive airway pressure permits heart- and lung-sparing mediastinal involved-site radiation therapy for patient with Hodgkin lymphoma in community clinic when deep inspiration breath-hold technique is not available. Pract Radiat Oncol. 2019;9:142–146. doi: 10.1016/j.prro.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Kil WJ, Pham T, Hossain S, Casaigne J, Jones K, Khalil M. The impact of continuous positive airway pressure on radiation dose to heart and lung during left-sided postmastectomy radiotherapy when deep inspiration breath hold technique is not applicable: A case report. Radiat Oncol J. 2018;36:79. doi: 10.3857/roj.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kil WJ, Pham T, Kim K. Heart sparing breast cancer radiotherapy using continuous positive airway pressure (CPAP) and conventional supine tangential fields: An alternative method for patients with limited accessibility to advanced radiotherapy techniques. Acta Oncol. 2019;58:105–109. doi: 10.1080/0284186X.2018.1503711. [DOI] [PubMed] [Google Scholar]
- 13.Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med. 2001;163:540–577. doi: 10.1164/ajrccm.163.2.9906116. [DOI] [PubMed] [Google Scholar]
- 14.Hoisak JDP, Sixel KE, Tirona R, Cheung PCF, Pignol J-P. Correlation of lung tumor motion with external surrogate indicators of respiration. Int J Radiat Oncol Biol Phys. 2004;60:1298–1306. doi: 10.1016/j.ijrobp.2004.07.681. [DOI] [PubMed] [Google Scholar]
- 15.Landberg T, Chavaudra J, Dobbs J, et al. Report 62. J ICRU. 1999;32:NP. [Google Scholar]
- 16.Neicu T, Ross B, John W, Steve BJ. Synchronized moving aperture radiation therapy (SMART): Improvement of breathing pattern reproducibility using respiratory coaching. Phys Med Biol. 2006;51:617. doi: 10.1088/0031-9155/51/3/010. [DOI] [PubMed] [Google Scholar]
- 17.Neicu T, Shirato H, Seppenwoolde Y, Jiang SB. Synchronized moving aperture radiation therapy (SMART): Average tumour trajectory for lung patients. Phys Med Biol. 2003;48:587–598. doi: 10.1088/0031-9155/48/5/303. [DOI] [PubMed] [Google Scholar]