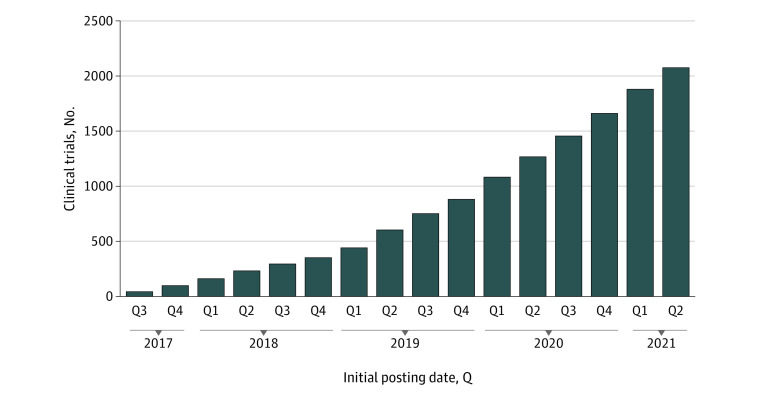
Figure. Cumulative US Clinical Trials With Informed Consent Forms Posted on ClinicalTrials.gov, by Initial Posting Date.
Numbers of registered trials by quarter (Q) from 2017 Q3 (n = 42) to 2021 Q2 (n = 2076). The 12 trials for which informed consent forms were posted from July 1 through 7, 2021 (ie, 2021 Q3), were omitted from this figure. The option to submit informed consent documents to ClinicalTrials.gov became available on June 29, 2017. Sponsors and investigators may submit informed consent forms to ClinicalTrials.gov for posting at any time during the study life cycle.