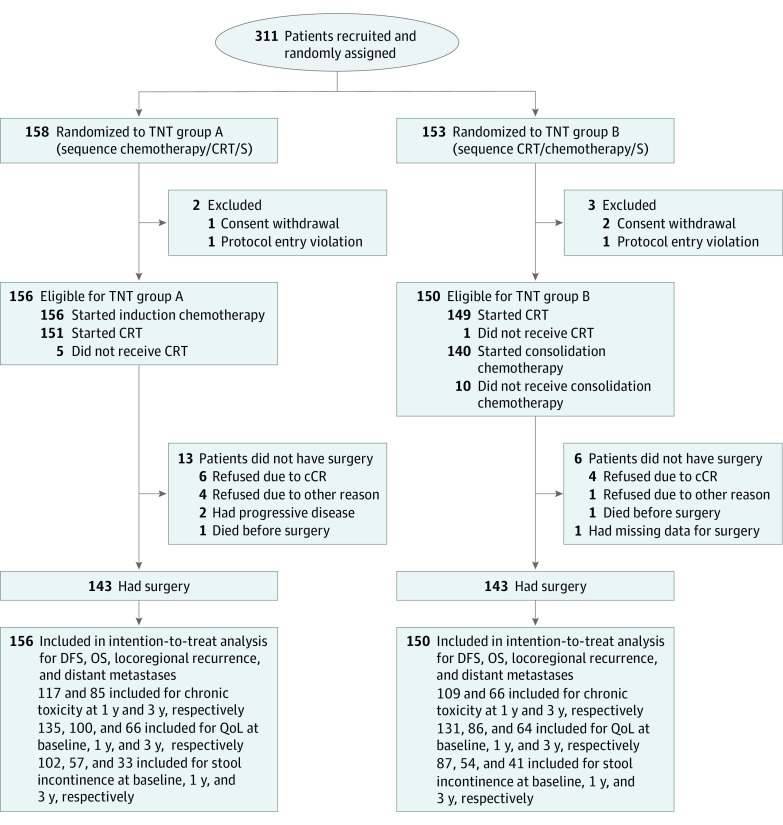
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Diagram.
Patients who were disease-free after protocol-specified treatment as well as patients with clinical complete response (cCR) who rejected surgery (S) and had nonoperative management were included in the assessment of toxicity and quality of life (QoL) assessment. For the assessment of stool incontinence, patients who were disease-free and stoma-free as well as patients with cCR who rejected surgery and had nonoperative management were included. CRT indicates chemoradiotherapy; DFS, disease-free survival; OS, overall survival; TNT, total neoadjuvant therapy.