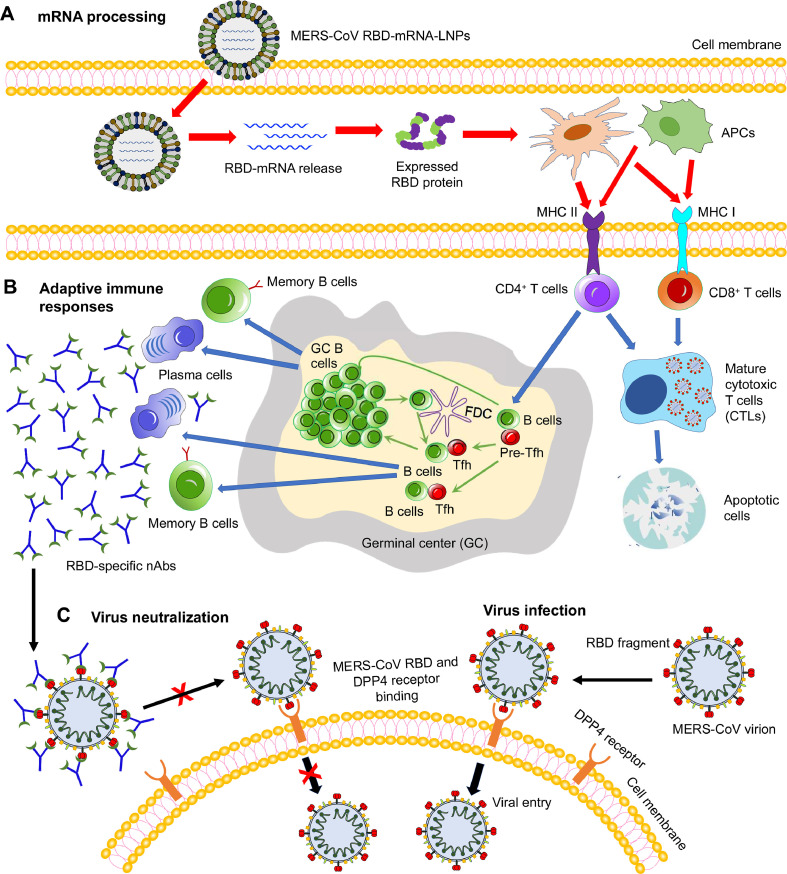
Fig 7.
Schematic view of the potential working model of MERS-CoV mRNA vaccines using RBD-mRNA as an example. (A) mRNA processing. Lipid nanoparticle (LNP)-encapsulated, nucleoside-modified MERS-CoV RBD-mRNA (pseudoU) was delivered into target cells, where it was released and then translated into the target receptor-binding domain (RBD) protein fragment. Subsequently, antigen-presenting cells (APCs), such as dendritic cells and macrophages, recognize the expressed protein, and present it via major histocompatibility complex I (MHC I) and MHC II molecules. (B) Adaptive immune responses. T (CD4+ or CD8+) and B cells were activated by interacting with the APCs. The activated CD8+ or CD4+ T cells either kill the virus-infected cells directly or help B cells generate antigen-specific antibodies, including neutralizing antibodies (nAbs). Germinal center (GC) B cells interact with T follicular helper (Tfh) and B cells to help promote the production of effective antibodies. (C) Virus infection and neutralization. In the absence of nAbs, MERS-CoV RBD binds its cellular receptor dipeptidyl peptidase 4 (DPP4) to enter target cells (virus infection). In the presence of MERS-CoV RBD-specific nAbs, nAbs bind to the RBD, blocking its binding to the DPP4 receptor and thus preventing subsequent MERS-CoV infection (virus neutralization). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)