Abstract
Opioid use disorders and fatal overdose due to consumption of fentanyl-laced heroin remain a major public health menace in the United States. Vaccination may serve as a promising potential remedy to combat accidental overdose and to mitigate the abuse potential of opioids. We previously reported the heroin and fentanyl monovalent vaccines carrying, respectively, a heroin hapten, 6-AmHap, and a fentanyl hapten, para-AmFenHap, conjugated to tetanus toxoid (TT). Herein, we describe the mixing of these antigens to formulate a bivalent vaccine adjuvanted with liposomes containing monophosphoryl lipid A (MPLA) adsorbed on aluminum hydroxide. Immunization of mice with the bivalent vaccine resulted in IgG titers of >105 against both haptens. The polyclonal sera bound heroin, 6-acetylmorphine, morphine, and fentanyl with dissociation constants (Kd) of 0.25 to 0.50 nM. Mice were protected from the anti-nociceptive effects of heroin, fentanyl, and heroin +9% (w/w) fentanyl. No cross-reactivity to methadone and buprenorphine was observed in vivo. Naloxone remained efficacious in immunized mice. These results highlighted the potential of combining TT-6-AmHap and TT-para-AmFenHap to yield an efficacious bivalent vaccine that could ablate heroin and fentanyl effects. This vaccine warrants further testing to establish its potential translatability to humans.
Introduction
The adulteration of heroin with more potent opioids such as fentanyl is an alarming public health menace amid the opioid crisis.1−3 Deaths due to heroin laced with synthetic opioids have been increasing since 2014 and have already outnumbered the deaths due to heroin use alone.4 Fentanyl is ∼30- to 50-fold more potent as an analgesic than heroin, and a smaller amount of the mixture can evoke the same euphoric effects of heroin alone, but with much lower production costs.5 Fentanyl is 5- to 20-fold more lethal than heroin (fentanyl LD50 = 1 to 3 mg/kg, heroin LD50 = 15 to 20 mg/kg),6,7 and the spiking of fentanyl in heroin exacerbates respiratory depression rates and brain hypoxia.8 Other more potent fentanyl analogues with potencies from 3- to 10,000-fold higher than morphine could also be used as potential adulterants.3,9,10 These dangerous drug combinations predispose the more than 500,000 Americans with heroin use disorder4,11 to an increased risk of fatal overdose due to consumption of adulterated heroin. Trafficking of adulterated heroin is not only a public health burden but also an issue of national security, as it puts law enforcement officers and first responders at risk of occupational exposure.12,13 The abuse of synthetic opioids has also been linked to an increased risk of acquiring an infectious disease such as the human immune deficiency virus (HIV) due to needle sharing.14,15 Finally, with the mounting estimated annual cost of ∼$78.5 billion in the United States16 incurred by the opioid epidemic, novel and pragmatic approaches are urgently needed to address this public health burden.
Limited treatment modalities remain a major barrier toward the successful mitigation of opioid use disorder. Evidence-based treatments,17 such as the use of methadone, buprenorphine, and naltrexone, while effective, are challenged by patient adherence rates and access to treatment facilities.18,19 In the absence of secondary protective measures, patients under this type of therapy who relapse from opioid use are poised for greater risk for overdose.19 While opioid overdose can be reversed by naloxone, a μ-opioid receptor antagonist,20 it has notable caveats: (1) multiple doses may be required to reverse the effects of synthetic fentanyl analogues;20,21 (2) naloxone has to be administered rapidly to victims shortly after being found unconscious, which may not always be realistic; and (3) naloxone precipitates opioid withdrawal symptoms and other complications.20,22
Current research efforts are focused on developing alternatives or complementary modalities to methadone, buprenorphine, naltrexone, and naloxone. Active immunization using opioid conjugate vaccines is an emerging potential therapeutic against opioid use disorder.23−26 Several research groups have been working on vaccines for drugs of abuse to methamphetamines,27 cocaine,28 oxycodone,29 heroin,30 and fentanyl and analogues.31−37 An opioid vaccine is composed of a molecule that structurally resembles the target opioid (a hapten) that is conjugated to an immunogenic carrier protein and formulated with an adjuvant that stimulates immune response.25,38 Immunization induces antibodies that target and sequester opioids in the blood and reduces the physiological effects of these opioids by preventing their access to the brain.24,25 Since the resulting antibody repertoire is highly selective to the target antigen, vaccines that target multiple opioids with very different chemical scaffolds, such as heroin and fentanyl, will require a combination of these monovalent vaccines.
Combination vaccines, also called polyvalent vaccines, that aim to raise immune response against multiple protein or polysaccharide antigens are not new,39 but combination vaccines that target small molecules such as opioids are relatively uncommon. Pravetoni et al. first reported the coformulation of a morphine and oxycodone vaccine.40 Our group has demonstrated that a combination heroin–HIV vaccine can successfully induce dual immune response against a heroin hapten and a peptide antigen.41 Recently, a vaccine that targets heroin and fentanyl has been reported and found to be efficacious in rodents and rhesus macaques.31,36,42 The use of a contiguous hapten that carries dual epitopes of heroin and fentanyl has also been proposed, but the study revealed several challenges that need to be addressed before it could be useful.43 Collectively, these studies suggest that mixing of two immunogens is a pragmatic and effective approach to induce dual immune response to structurally diverse opioids.
We recently reported the synthesis of heroin and fentanyl haptens (Figure 1), their conjugation to tetanus toxoid (TT) carrier protein, and their formulation as monovalent vaccines.30,44 The heroin hapten (6-AmHap) is a hydrolytically stable compound that has been shown to protect rodents from heroin-induced effects.30 The fentanyl hapten (para-AmFenHap) is composed of the intact fentanyl scaffold and uses the para position at the phenethyl ring as the linker attachment site.44 In this study, we report the formulation and immune responses to animals of a bivalent vaccine composed of TT-6-AmHap and TT-para-AmFenHap. These antigens were coformulated with an adjuvant comprising Army Liposome Formulation (ALF)45 with monophosphoryl lipid A and 43% cholesterol, otherwise called ALF43, and adsorbed to aluminum hydroxide (ALF43A). To test this formulation, we immunized mice with (TT-6-AmHap + TT-para-AmFenHap)/ALF43A vaccine and evaluated immunogenicity and efficacy. We found vaccine-induced high-affinity antibodies against heroin, 6-acetylmorphine (6-AM), morphine, and fentanyl, which protected mice against heroin and fentanyl-induced effects. The following are the novel aspects of this work: (1) This is the first report of a bivalent heroin-fentanyl vaccine that is adjuvanted with liposomal monophosphoryl lipid A. (2) This is the first report of antibody affinity at different time points postimmunization. (3) This is the first report of an in vivo cross-reactivity study of a bivalent heroin–fentanyl vaccine against opioid receptor agonists (methadone and buprenorphine) and antagonist (naloxone). These results demonstrated the feasibility of a practical vaccine against fentanyl and warrants further development for clinical testing.
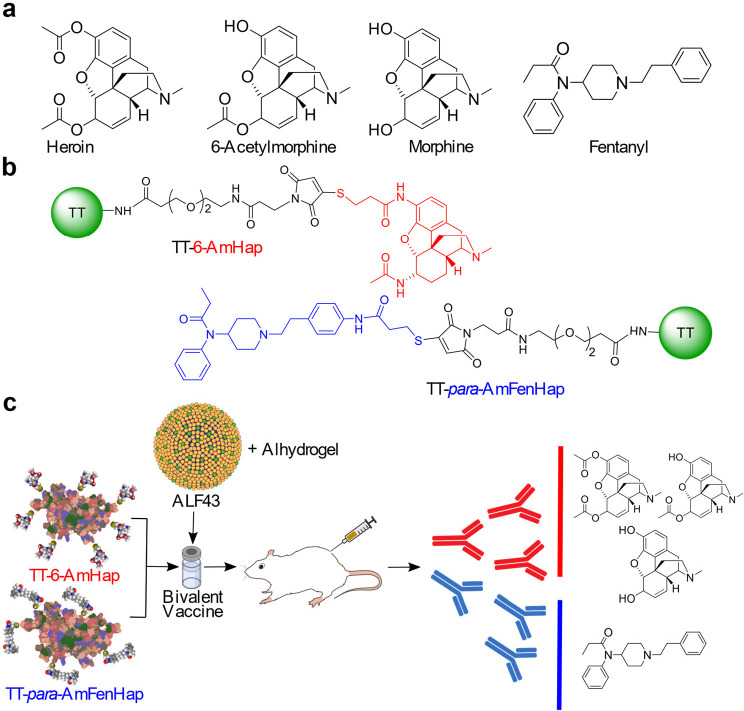
Figure 1.
Structure of drugs and conjugates and the research strategy implemented in this study. (a) Chemical structures of heroin, 6-AM, morphine, and fentanyl. (b) Structures of TT-6-AmHap and TT-para-AmFenHap conjugates. The 6-AmHap and para-AmFenHap haptens and the NHS-(PEG)2-maleimide cross-linker [SM(PEG)2] linker are depicted in red, blue, and black, respectively. (c) Research strategy: TT-6-AmHap (10 μg) and TT-para-AmFenHap (10 μg) conjugates were mixed with ALF43 and Alhydrogel (ALF43A) adjuvant, and injected i.m. to female Balb/c mice. Adjuvant doses were the same in monovalent and bivalent formulations. The ability of the serum IgG to sequester the opioids in vitro and block their anti-nociceptive effects in vivo was tested.
Results
Immune Responses to 6-AmHap and para-AmFenHap
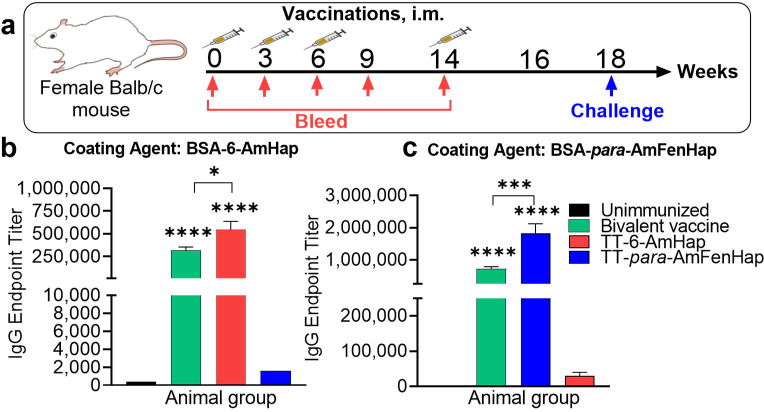
The week 16 mice sera from the bivalent vaccine group showed IgG end point titers of 716,800 and 318,588 against para-AmFenHap and 6-AmHap, respectively, compared to unvaccinated mice that had IgG end point titer of <400 for either hapten (Figure 2). Monovalent vaccine groups gave 1,820,444 and 546,133, for TT-para-AmFenHap/ALF43A (fentanyl vaccine) and TT-6-AmHap/ALF43A (heroin vaccine), respectively. Fentanyl vaccine-induced sera gave an end point titer of 1600 against off-target BSA-6-AmHap ELISA coating agent. Heroin vaccine-induced sera gave an end point titer of 30,222 against the off-target BSA-para-AmFenHap ELISA coating agent. The % cross-reactivity was calculated by the ratio of the off-target antigen to the target antigen.40 Monovalent heroin and fentanyl vaccines had 5.53% and 0.088% cross-reactivity, respectively.
Figure 2.
Immune response to haptens. Mice (n = 10 per group) were immunized at weeks 0, 3, 6, and 14 with the monovalent or bivalent vaccine formulation. Monovalent formulation used 10 μg of antigen. Bivalent vaccine was composed of 10 μg TT-para-AmFenHap and 10 μg TT-6-AmHap. Immunogenicity was evaluated on sera collected on week 16 using ELISA with the indicated coating antigen: (a) animal study timeline; hapten-specific IgG end point titers to (b) BSA-6-AmHap and (c) BSA-para-AmFenHap. Data shown are mean ± SEM. Statistical differences were tested using Mann–Whitney nonparametric t-test (**** p < 0.0001; **, p < 0.005; *, p < 0.05).
Serum Binding of Fentanyl, Heroin, and Metabolites
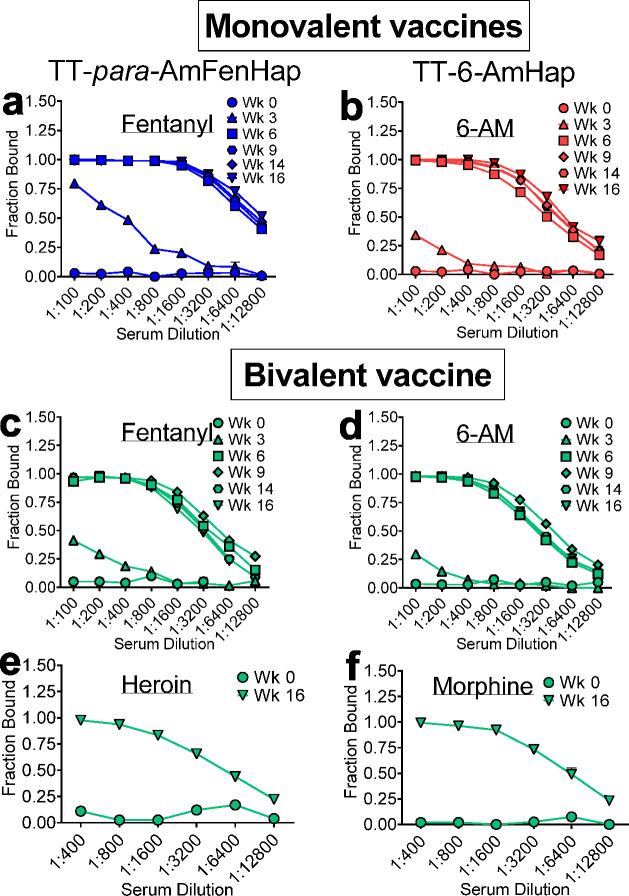
Serum sequestration of the target opioids 6-AM and fentanyl was evaluated using ED46 at different weeks post-immunization. The week 3 sera had marginal binding capacity against fentanyl in both monovalent and bivalent vaccine groups. Binding capacity increased in week 6 and became comparable at weeks 9, 14, and 16 in either monovalent or bivalent vaccine groups (Figure 3a,c). A similar trend was observed for 6-AM binding (Figure 3b,d).
Figure 3.
Serum sequestration of drugs in vitro. Pooled serum samples from indicated weeks were diluted with a buffer that contained 5 nM of indicated drugs and dialyzed against buffer in an equilibrium dialysis plate. Sodium fluoride (3–4 mg/mL) was added to the buffer to impede heroin degradation. Drug concentrations in the sample and buffer chambers were determined after 24 h, and fraction bound was calculated. Serum binding from monovalent immunization: (a) Fentanyl from TT-para-AmFenHap group and (b) 6-AM from TT-6-AmHap group. Serum binding from bivalent immunization: (c) fentanyl, (d) 6-AM, (e) heroin, and (f) morphine. Data shown are mean ± SEM of triplicate determinations.
Two weeks prior to the drug challenge, the week 16 sera were also assayed for binding against heroin and morphine (Figure 3e,f). Both drugs were effectively bound at high serum dilutions (fraction bound ≥0.50 from 1:400 to 1:6400). When tested for the ability to bind a mixture of heroin + 9% (w/w) fentanyl, week 16 sera showed significant binding of both drugs in the mixture (SI Figure S2).
Temporal Affinity Maturation of Vaccine-Induced Antibodies
The average Kd values of individual and bivalent vaccine-induced antibodies from pooled sera at weeks 3, 6, 9, 14, and 16 were measured by ED.46 The competitive inhibition curves and IC50 values are shown in SI Figure S3. Monovalent heroin vaccine-induced antibodies had low antibody affinity after 3 weeks, but significantly increased affinity (decreased Kd values) plateauing 6 weeks after the first dose (Figure 4a). This result was paralleled by monovalent fentanyl vaccine-induced antibodies which gave gradual improvement in antibody affinity as demonstrated by decreasing Kd values (Figure 4b). A similar trend in antibody affinity was observed in bivalent vaccine for both drugs. Only the week 3 sera differed significantly between monovalent fentanyl and bivalent vaccines. The week 16 sera from the bivalent vaccine group showed subnanomolar affinities against fentanyl, heroin, and metabolites. Heroin and fentanyl had Kd values of 0.25 ± 0.16 nM and 0.33 ± 0.06 nM, respectively. The heroin metabolites, 6-AM, and morphine had Kd values of 0.41 ± 0.15 nM and 0.50 ± 0.24 nM, respectively. No significant difference in Kd between these drugs was observed.
Figure 4.
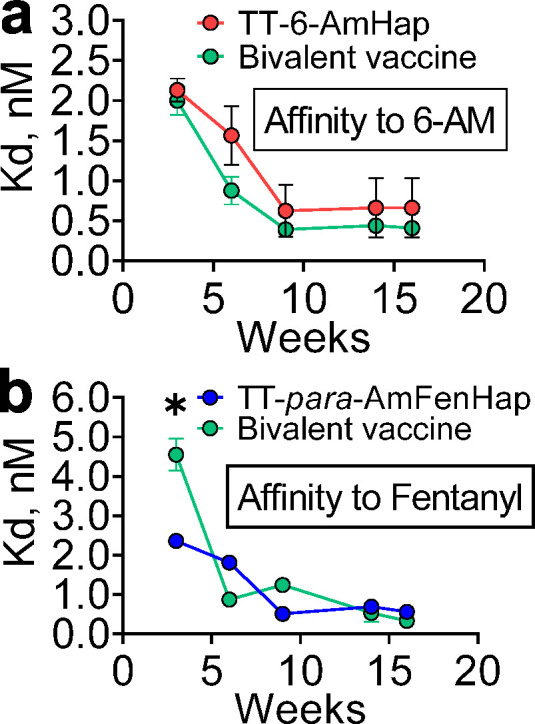
Temporal antibody affinity maturation. Pooled serum samples at indicated weeks from mice immunized with monovalent or bivalent vaccines were diluted with a buffer that contained 5 nM of isotopically labeled tracer and dialyzed against buffer in an ED plate. Drug concentrations in the sample and buffer chambers were determined after 24 h, and fraction bound was calculated: (a) TT-6-AmHap monovalent and bivalent vaccine-induced antibodies binding to 6-AM, (b) TT-para-AmFenHap monovalent and bivalent vaccine-induced antibodies binding to fentanyl. The Kd values were calculated as detailed in the Methods section. Data shown are mean ± SEM of triplicate determinations. Statistical significance was determined using the two-tailed, unpaired t test. *, p < 0.05.
Vaccine Efficacy against Heroin and Fentanyl Challenge
Mice were challenged s.c. at week 18 with increasing doses of heroin (0.05 to 4 mg/kg). Fifteen minutes after each heroin dosing, thermal nociception was measured using a hotplate test. Immunized mice became unresponsive to thermal stimuli (100% MPE) starting at ∼1 mg/kg heroin, compared to non-immunized mice which reached 100% MPE at ∼0.5 mg/kg heroin (Figure 5a). The ED50 values for non-immunized and immunized mice were 0.20 ± 0.02 mg/kg and 0.70 ± 0.09 mg/kg, respectively. This translated to an ED50 shift of ∼3.5-fold (Figure 5d).
Figure 5.
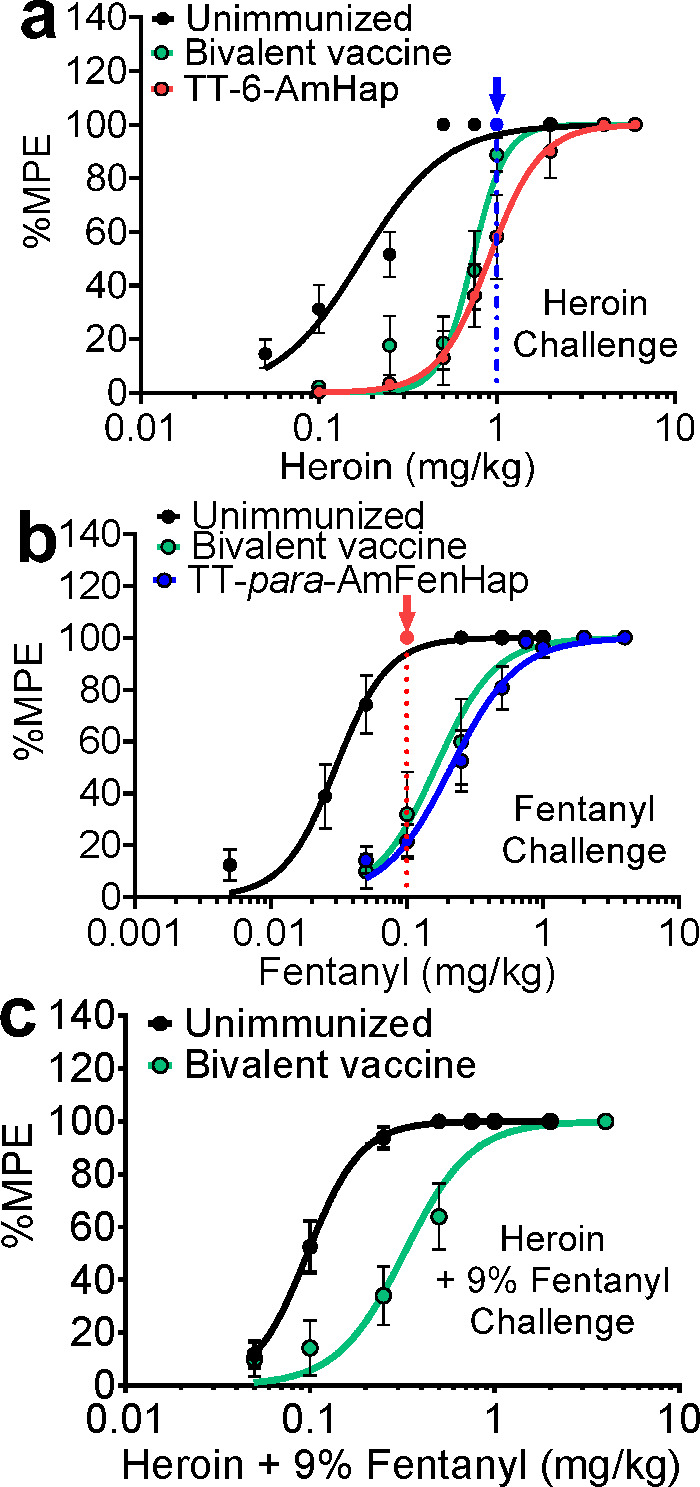
Vaccine efficacy against heroin and fentanyl-induced thermal anti-nociception. Mice (n = 10 per group) were challenged s.c. with an increasing dose of either heroin, fentanyl, or 9% (w/w) fentanyl in heroin mix. The anti-nociceptive effects were assessed by hotplate assay 15 min after drug dosing and reported as %MPE: (a) heroin challenge, (b) fentanyl challenge, and (c) heroin + 9% (w/w) fentanyl challenge. Data shown are mean ± SEM. Blue and red arrows depict mice (n = 10) challenged with a single dose of either heroin or fentanyl to measure in vivo cross-reactivity.
Mice also were challenged s.c. at week 18 with increasing doses of fentanyl (0.005 to 4 mg/kg). Fifteen minutes after each fentanyl dosing, thermal nociception was measured using a hotplate test. Very low doses of fentanyl were required to produce anti-nociception in mice. Non-immunized mice reached 100% MPE at ∼0.05 mg/kg fentanyl compared to immunized mice that achieved 100% MPE at a 10-fold higher dose of ∼0.50 mg/kg fentanyl (Figure 5b). The ED50 values for non-immunized and immunized mice were 0.03 ± 0.01 mg/kg and 0.20 ± 0.05 mg/kg, respectively. This translated to an ED50 shift of ∼6.7-fold (Figure 5e).
Mice were challenged s.c. at week 18 with increasing doses of 9% fentanyl in heroin (0.05 to 4 mg/kg). The 9% (w/w), i.e., 1:10, dose ratio of fentanyl to heroin was used a benchmark to mimic the circulating adulterated heroin in the illicit market.8,10 Fifteen minutes after each heroin–fentanyl mix dosing, thermal nociception was measured using the hotplate assay. Immunized mice reached 100% MPE at ∼0.75 mg/kg heroin (containing ∼0.075 mg/kg fentanyl), compared to non-immunized mice that reached 100% MPE at ∼0.25 mg/kg heroin (containing ∼0.025 mg/kg fentanyl) (Figure 5c). The ED50 values for non-immunized and immunized mice were 0.10 ± 0.01 mg/kg and 0.34 ± 0.05 mg/kg, respectively. This translated to an ED50 shift of ∼3.4-fold (Figure 5f).
Individual vaccine formulations were tested for in vivo cross-reactivity against heroin and fentanyl. When challenged with 1.0 mg/kg heroin s.c., mice that were immunized with TT-para-AmFenHap/ALF43A (fentanyl vaccine) reached 100% MPE and were not statistically different from the non-immunized control animals (Figure 5a, blue arrow). Similarly, mice that were immunized with the TT-6-AmHap/ALF43A (heroin vaccine) reached 100% MPE and were not statistically different from the non-immunized control animals, when challenged with ∼0.1 mg/kg fentanyl, s.c. (Figure 5b, red arrow).
In Vivo Cross-Reactivity to Opioid Receptor Agonists
The vaccine-induced antibodies cross-reacted with therapeutic opioid receptor agonists was evaluated in vivo. Mice were immunized with the bivalent vaccine and challenged with an increasing cumulative dose of either buprenorphine or methadone (Figure 6a,b). As expected, non-immunized control mice showed increasing %MPE values with increasing doses of either opioid receptor agonist. No significant difference was observed when compared with immunized mice.
Figure 6.
Cross-reactivity of bivalent vaccine to therapeutics in vivo. Mice (n = 10 per group) were challenged s.c. with increasing doses of either buprenorphine or methadone. The anti-nociceptive effects were assessed by hotplate assay 15 min after drug dosing and reported as %MPE: (a) buprenorphine, (b) methadone, and (c) experimental flow to assess the cross-reactivity of naloxone in vivo. Mice (n = 10 per group) were challenged s.c. with 0.50 mg/kg heroin with 0.05 mg/kg fentanyl; 15 min afterwards, mice received 0.1 mg/kg naloxone, s.c. The thermal nociception was assessed 5 and 20 min post-naloxone. (d) Anti-nociceptive effects of heroin + 9% (w/w) fentanyl in the presence or absence of naloxone. Data shown are mean ± SEM. Statistical significance (naloxone vs saline group) was determined using a two-tailed, unpaired t-test. ***, p < 0.0005, ****, p < 0.0001. Nlx, naloxone, Sal, saline.
In Vivo Cross-Reactivity to Naloxone
Mice were challenged with 0.5 mg/kg heroin containing 0.05 mg/kg fentanyl, s.c., and then administered 0.1 mg/kg naloxone s.c. 15 min post-challenge dose. This dose was chosen because it gave suboptimal %MPE in the immunized mice based on the dose–response curve from Figure 5. The thermal nociception was assessed 5 and 20 min post-naloxone dosing. No significant difference in the %MPE values was observed between control and immunized mice (Figure 6c).
Discussion
The presence of trace amounts of fentanyl and analogues in the heroin supply has greatly contributed to the increased number of overdose deaths.3−5,10 We addressed this public health issue by formulating a bivalent vaccine against heroin and fentanyl using the TT-6-AmHap and TT-para-AmFenHap conjugates previously.30,44 In the present study, we found that (1) immunization with a bivalent vaccine that contained TT-6-AmHap and TT-para-AmFenHap induced high anti-hapten IgG titers; (2) the serum IgG strongly bound heroin, 6-AM, morphine, and fentanyl in vitro with nanomolar affinity; and (3) immunization protected mice against thermal anti-nociception induced by heroin, fentanyl, and heroin + 9% (w/w) fentanyl challenges.
We previously reported the development of monovalent TT-6-AmHap and TT-para-AmFenHap vaccines.30,44 The same animal species, immunization regimen, and adjuvant formulation were used. In these previous works, we obtained >105 hapten-specific IgG end point titers for either TT-6-AmHap30 or TT-para-AmFenHap.44 In the present study, immunization with bivalent vaccine induced high hapten-specific IgG end point titers confirming that distinct population of antibodies were generated. Very little cross-reactivity (<6%) was observed between the antibodies induced by monovalent vaccines (Figure 2b,c). We believe that this value is too small and in fact did not manifest in vivo when mice were challenged with heroin or fentanyl (Figure 5a, blue arrow; b, red arrow).
Mixing two distinct antigens could influence the resultant IgG end point titers. The end point titers of bivalent vaccine groups were significantly lower than those of the monovalent vaccine groups (Figure 2b,c. Our data paralleled that of Hwang et al.31 who reported a heroin–fentanyl admixture mixture composed of 25 μg each of individual antigens. When compared with 50 μg of individual antigens, the admixture vaccine gave significantly lower end point titers.31 In a separate report from the same group,42 individual vaccines were added with unconjugated carrier protein (i.e., 50 μg conjugate + 50 μg unconjugated carrier) such that all groups received the same total carrier protein amount (100 μg). This resulted in higher end point titers observed in the admixture than in the individual vaccines.42 Pravetoni et al.40 reported similar findings in a coadministered morphine and oxycodone vaccine. When the carrier protein amount was normalized among monovalent and bivalent vaccines by adding unconjugated carrier protein in the monovalent formulations, higher anti-hapten end point titers were observed in the bivalent vaccine. In our study, we used 10 μg of the fentanyl vaccine and 10 μg of the heroin vaccine to make a bivalent formulation (20 μg), and no normalization by equalizing the total protein was employed.
Others have also recognized the change in titers between monovalent and bivalent vaccine formulations using different adjuvants and administration route.31,40,42,47 Pravetoni et al.40 observed that IgG end point titers of a morphine–oxycodone bivalent vaccine formulated with Freund’s adjuvant had higher IgG end point titers compared to morphine or oxycodone vaccine alone when administered intraperitoneally (i.p.). The same group compared the immunogenicity and efficacy of a bivalent nicotine vaccine composed of distinct nicotine haptens47 where no significant difference in titer was observed when vaccines were adjuvanted with alum and injected s.c. However, higher titers were obtained in monovalent vaccines. Freund’s adjuvant was used and administered i.p. In the context of a heroin-fentanyl vaccine with CpG and alum as adjuvants, Hwang et al.31,42 reported that bivalent vaccine formulation administered s.c. yielded higher IgG end point titers than the corresponding heroin or fentanyl alone. These seminal studies suggested that the degree of humoral response is influenced not only by the dose but also by the number of distinct immunogens present, adjuvant in the formulation, and administration route. How this happens immunologically remain unclear, and this could be a fertile ground for future research.
The immune response to specific antigens could be maximized using potent adjuvants that may induce an antigen dose-sparing effect in vaccines for infectious diseases.48,49 This suggests that at a given adjuvant dose, a ceiling amount of IgG could be induced where the amount of antigen used may have little effect, at a certain range, on the magnitude of immune response. This is consistent with our observation that sera from mice immunized with the bivalent vaccine yielded lower IgG end point titer compared with the fentanyl monovalent formulation. The monovalent and bivalent formulations contained the same adjuvant dose (20 μg of synthetic MPLA in ALF43, and 30 μg aluminum in aluminum hydroxide) but different total amounts of antigen (monovalent = 10 μg, bivalent = 20 μg). These results showcased the high potency of ALF43A adjuvant in inducing an immune response to opioid-based antigens.
The goal of immunization was to induce IgG that could sequester opioids in the blood and prevent their access to the brain.24−26,38In vitro, the ability of the sera to sequester opioids may be predictive of in vivo sequestration.44,46 We found that the sera from mice immunized with the bivalent vaccine effectively sequestered heroin, 6-AM, morphine, and fentanyl (Figure 3). We previously reported that immunization using TT-6-AmHap alone induced IgG that did not cross-react with fentanyl owing to its distinct difference from the structure of the 6-AmHap hapten.30 In this study, we showed that this paralleled the in vivo data (Figure 6). These results confirmed that (1) the selectivity of the vaccine-induced IgG was retained in the bivalent vaccine and (2) dual immunogenic response has been achieved. These findings are consistent with bivalent vaccines reported previously where individual vaccines were coadministered.31,40−42,47 Driven by these results, it is prudent to anticipate that the broad specificities of IgG induced by TT-6-AmHap30 and the TT-para-AmFenHap44 monovalent vaccines against related drugs of abuse might also be retained in the bivalent formulation.
The efficacy of opioid vaccines could stem from the binding strength and concentration of the generated opioid-specific IgG. We used competition ED46 to determine the average Kd of the polyclonal sera. We found that sera from bivalent vaccine-immunized mice had very strong affinity (Kd < 5 nM) against all drugs tested (heroin, 6-AM, morphine, and fentanyl). When compared with monovalent vaccines, a similar trend in Kd values was found. Nanomolar affinity could mean a longer duration of the antibody–antigen complex from minutes to hours50 suggesting that once bound by IgG in the blood, drugs would very slowly dissociate preventing redistribution to tissues such as the brain. The ability of IgG to bind heroin metabolites 6-AM and morphine is crucial, because the physiological effects of heroin are mainly mediated by these molecules owing to the short half-life (∼3 to 4 min) of heroin in the blood.38
Bivalent vaccine-induced antibodies did not cross-react with selected therapeutic drugs in vitro and in vivo. We previously reported that monovalent vaccine-induced antibodies did not cross-react with therapeutic drugs in vitro.30,44 In this study, we reported that this is also true in vivo. Using the full and partial opioid receptor agonists, methadone and buprenorphine, respectively, we observed that the anti-nociceptive responses of mice immunized with the bivalent vaccine did not differ significantly from those of non-immunized mice (Figure 6). Similarly, naloxone rescue experiments demonstrated that immunization using the bivalent vaccine did not impede naloxone. These therapeutic drugs are structurally distinct from the haptens, which may explain why vaccine-induced antibodies did not bind these drugs. These results suggested that the bivalent vaccine could be used as a complement of existing therapeutic drugs to opioid use disorders.
Opioids reduce pain sensation as a result of binding to opioid receptors in the brain.25 This brain-mediated nociception was exploited as a surrogate of vaccine efficacy using a hotplate assay. The use of the hotplate to evaluate the vaccine performance has consistently produced reproducible behavioral changes in response to the challenge drugs.30,51 Mice that received the bivalent vaccine were protected against repeat fentanyl and heroin challenges (s.c.) (Figure 5). We found the following doses to be equipotent (based on ED50) in non-immunized mice: fentanyl (0.03 ± 0.01 mg/kg), heroin (0.20 ± 0.02 mg/kg), and 10% fentanyl in heroin (0.10 ± 0.01 mg/kg heroin containing 0.01 mg/kg fentanyl). This confirmed that the presence of fentanyl dramatically enhances the potency of heroin. For example, the estimated lethal dose of fentanyl and heroin in humans is ∼2 mg (∼0.29 mg/kg, assuming 70 kg human) and ∼50 mg (∼0.72 mg/kg, assuming 70 kg human), respectively, but when mixed, as in adulterated street heroin, these lethal doses are dramatically reduced. The human lethal dose of fentanyl-laced heroin is currently unknown, but a study suggested that the addition of fentanyl to heroin at a 1:10 (by weight) can more easily cause death through respiratory depression and brain hypoxia than the individual opioids.8
The protection level observed in this study agrees with previous work that used other assays to demonstrate vaccine efficacy.30,44 Our findings also concur with other works showing that fentanyl-specific antibodies generated from a fentanyl–hapten conjugate decreased anti-nociception and opioid seeking behaviors to fentanyl33−35,44 and a heroin–fentanyl mix31,36,42 in behavioral assays in both rodents and nonhuman primates. Despite the disadvantage of animals learning “escape behaviors” as study end points upon repeated testing,52,53 the challenge drugs reliably shifted the dose–effect curves to higher doses in the immunized animals. The results from this study show a promising avenue for opioid vaccine research and the potential for moving toward clinical trial in efforts to combatting the rise of opioid use disorder in the U.S.
The components of our vaccine formulation are potent, safe, and easily translatable to the clinic. The TT carrier protein is highly immunogenic, which is desirable in the context of T-cell-dependent antigen recognition. The ALF43A adjuvant contains two powerful immunostimulants: monophosphoryl lipid A, an agonist of Toll-like Receptor-4 (TLR-4), a hallmark of innate immune cells, and aluminum hydroxide that is thought to trigger inflammasome activation.54 ALF43A has been shown to induce high immune response in a myriad of antigens from opioids to infectious diseases like HIV-1 and malaria.45 Together, these vaccine components could act on multiple pathways at the interface of the innate and adaptive immune system, which resulted in high immunogenicity of the bivalent vaccine.
In summary, we have described a unique and pragmatic vaccine to combat heroin adulterated with fentanyl. Our heroin hapten resembles a heroin molecule with hydrolyzable ester groups replaced by amides,30 suggesting that this may ensure the integrity of the vaccine during storage. Owing to its structure, the fentanyl hapten para-AmFenHap is also relatively stable.44 The carrier protein used for conjugation is a U.S. Food and Drug Administration (FDA)-licensed vaccine against tetanus infection.55 The conjugation chemistry is a facile and reproducible thiol–maleimide reaction that has been widely used in the pharmaceutical industry. This reaction consistently yields at least 30 haptens per carrier molecule, a pivotal factor in conjugate vaccine design because it influences efficacy.51 Importantly, the bivalent vaccine uses an adjuvant that is proven safe and effective in the context of vaccines against many diseases.45,56 We have also shown that the IgG induced by these immunogens did not cross-react with drugs used for opioid abuse management therapies in vivo, which highlighted the potential of this bivalent vaccine as a potential adjunct to existing evidence-based therapies to serve as secondary protection to patients undergoing addiction treatments. The following future studies are warranted: (1) investigation of the efficacy of this proposed vaccine against the respiratory depressive effects of opioids; (2) measurement of how opioid distributions in the blood and brain change in vaccine recipient animals; and (3) evaluation of how this vaccine modulates reward-seeking behaviors in self-administration models. The immunogenicity and efficacy data presented in this work showcase the seminal attributes of a potential vaccine against heroin and fentanyl that is worthy of further development toward translation to humans.
Methods
The list of materials used and their sources are provided in the SI Supplementary Methods.
Synthesis and Characterization of Vaccine Antigens
The heroin hapten, 6-AmHap, and the fentanyl hapten, para-AmFenHap, were separately coupled to the tetanus toxoid (TT) carrier protein using the optimized coupling method as previously described.44,51,57,58 Briefly, TT was incubated with the NHS-(PEG)2-maleimide cross-linker [SM(PEG)2] cross-linker at a 1:1600 molar ratio for 2 h. Excess linker was removed using a Zeba spin column, and the protein content of the eluate was quantified using a bicinchoninic acid (BCA) assay kit. The excess haptens were removed by repeated dialysis against phosphate buffered saline (PBS), pH 7.4, at 4 °C. The TT–hapten conjugates were sterile filtered and quantified using the BCA protein assay. The number of haptens attached per TT molecule in each conjugate was assessed using matrix assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, and about 30–35 haptens per TT molecule were consistently obtained.30,44
Vaccine Formulation
The vaccines contained TT–hapten conjugates and a liposomal adjuvant. The adjuvant was composed of Army Liposome Formulation with 43% cholesterol adsorbed to aluminum hydroxide as described.59,60 ALF43 consisted of the following lipids: 1,2-dimyristoyl-sn-glycero-3-phosphoglycerol (DMPG), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), synthetic MPLA (3D-PHAD), and cholesterol. Briefly, ALF43 was prepared from a mixture of DMPC:cholesterol:DMPG in a molar ratio of 9:7.5:1, with the addition of 3D-PHAD. The molar ratio of phospholipid: 3D-PHAD was 8.8:1. The liposomal adjuvant was a lyophilized powder derived from small unilamellar vesicles. To make the bivalent vaccine, the lyophilized ALF43 was mixed with 10 μg TT-6-AmHap and 10 μg TT-para-AmFenHap, 30 μg of aluminum in aluminum hydroxide (Alhydrogel), and 20 μg 3D-PHAD per dose of 50 μL in PBS, pH 7.4. Monovalent vaccines used either 10 μg TT-6-AmHap or 10 μg TT-para-AmFenHap and the same adjuvant dose as the bivalent vaccine.
Animal Studies
All animal studies were conducted under an approved animal use protocol in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALACi)-accredited facility in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals. Experiments involving animals adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals, 8th edition.61 Briefly, ∼7-week-old female Balb/c mice (n = 10 per vaccine group) (Jackson Laboratories, Bar Harbor, ME) were immunized via intramuscular (i.m.) route in alternate rear thighs with 50 μL of vaccine formulation on weeks 0, 3, 6, and 14. Mice were bled at weeks 0, 3, 6, 9, and 16.
Nociception Assays
The hotplate assay was used to assess vaccine efficacy in mice at week 18 as previously described.44,62 Briefly, a cumulative dose–response to heroin, fentanyl, and heroin + 9% fentanyl dissolved in saline was carried out via the subcutaneous (s.c.) route. Baseline hotplate latencies to the thermal stimulus were determined at 56 °C by gently placing the mice on a hotplate with a cylindrical cage to prevent escape, followed by the administration of the challenge drugs and post-challenge testing every 15 min. The licking, lifting, shaking of the hind paws, or jumping from the hot surface was used as the study end point with a cutoff latency set to 30 s to prevent tissue damage. The anti-nociceptive effects were quantified in terms of % maximum possible effect (%MPE) according to eq 1
| 1 |
Challenge experiments were performed at week 18 via s.c. route of administration, using increasing doses of heroin•HCl, fentanyl•HCl, or a 9% mixture of fentanyl•HCl and heroin•HCl. All drugs were solubilized in 0.9% saline. Anti-nociceptive effects were assessed 15 min after each challenge drug injection. The following dose regimen was used: fentanyl: 0.05, 0.10, 0.25, 0.50, 0.75, 1.00, 2.00, 4.00, 6.00 mg/kg; heroin: 0.10, 0.25, 0.50, 0.75, 1.00, 2.00, 4.00 mg/kg; and heroin + 9% fentanyl: 0.050 + 0.0050, 0.10 + 0.010, 0.250 + 0.025, 0.50 + 0.050, 0.75 + 0.075, 1.00 + 0.10, 2.0 + 0.20, 4.0 + 0.4, respectively (mg/kg). For methadone challenge experiments, the following doses were used: 0.125, 0.250, 0.500, 1.000, 1.500, 2.000, 2.500, and 5.000 mg/kg. For the buprenorphine challenge experiment, the following doses were used: 0.0125, 0.025, 0.050, 0.100, 0.150, 0.200, 0.250, 0.500, and 0.750 mg/kg. For the naloxone challenge experiment, mice were challenged with 0.50 mg/kg heroin combined with 0.050 mg/kg fentanyl, then 0.10 mg/kg naloxone 15 min later. Anti-nociception was assessed 5 and 20 min post-naloxone treatment.
ELISA
The IgG end point titers against haptens were quantified from mice sera at week 16. Plates were coated with either BSA-6-AmHap, BSA-para-AmFenHap, or TT (0.1 μg/0.1 mL in PBS, pH 7.4) and incubated overnight at 4 °C. ELISA was performed the next day as noted.30,44,51
Serum Binding Measurements
Serum binding was measured using equilibrium dialysis (ED) as noted.44,46 Mouse sera from week 16 were diluted with 0.05% BSA in PBS, pH 7.4 (ED buffer), containing 5 nM of a drug. For heroin serum binding analysis, the ED buffer was added with 3–4 mg/mL of sodium fluoride (NaF) to prevent ester-mediated hydrolysis.63 An aliquot (100 μL) was seeded into the sample chambers of a rapid ED plate, and the buffer chamber was filled with 300 μL of ED buffer. The plate was incubated at 4 °C and 300 rpm for 24 h in a thermomixer. Aliquots (90 μL) from sample and buffer chambers were pipetted out and analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS).
Sera were diluted to limit nonspecific binding and to permit multiple measurements from limited serum samples.46 This was acceptable given that the end point titers measured were sufficiently high. Pre-immune (week 0) and post-immune (week 16) sera were diluted with 5 nM of either heroin, fentanyl, 6-AM, or morphine in ED buffer and dialyzed against buffer for 24 h using a semipermeable membrane with a molecular weight cutoff of 12 kDa. When the ED buffer was added with 3–4 mg/mL of NaF, degradation was adequately suppressed to permit intact heroin measurements at dilutions tested (SI, Figure S1). Dilutions were chosen (1:400 to 1:51,200), such that 100% of the drug was bound at the initial concentration of 5 nM.
Determination of Antibody Affinity (Kd)
The Kd of anti-hapten IgG in serum was measured using competition ED as noted.44,46 Briefly, mouse sera were diluted with 5 nM of isotopically labeled tracer drug (dx, where x = number of heavy isotopes) in ED buffer at a serum dilution that yielded 50% fraction bound in the binding experiments. The buffer chambers were filled with ED buffer that contained an increasing concentration of the competitor drug (final concentration, 0 nM to 40 nM). Half-maximal inhibitory concentration (IC50) was interpolated using a four-parameter logistic curve (plot of % inhibition vs concentration of competitive inhibitor). The % inhibition values were obtained using eq 2 and were used to calculate Kd according to eq 3:46
| 2 |
Where [dx]bound, I = [dx]sample chamber – [dx]buffer chamber; [dx]bound, I0= concentration of the dx-tracer in the absence of competitive inhibitor.
| 3 |
Where [I50] = molar concentration of the competitive inhibitor required for 50% inhibition; [Tt] = total molar concentration of dx-tracer after equilibrium (typical value is 1.25 nM); b = fraction of bound dx-tracer in the absence of competitive inhibitor.
LC-MS/MS
A binary ultraperformance liquid chromatograph coupled to a triple quadrupole tandem mass spectrometer (LC-MS/MS) operated in multiple reaction monitoring (MRM) mode was used to quantify drug concentrations.44,46 The gradient information, mass spectrometry parameter settings, and MRM transitions are provided in the SI Supplementary Methods.
Data Analysis
GraphPad Prism 8 (GraphPad Software, La Jolla, CA) was used for all statistical analyses and graphing of data. Data were analyzed using Student’s unpaired two-tailed t-test. Differences were considered significant if p ≤ 0.05. For the dose–response studies, a nonlinear regression analysis (four-parameter logistic curve) was used to generate best-fit line.64 All values represent the mean ± standard error of the mean (SEM).
Acknowledgments
This work was supported by the National Institutes of Health through the NIH HEAL Initiative under award numbers UG3DA048351 and Avant Garde Award NIH 1DP1DA034787-01 to GRM. The work of GRM, OBT, ZB, EK, CW, and RCB was supported through a Cooperative Agreement Award (no. W81XWH-07-2-067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine and the U.S. Army Medical Research and Medical Command (MRMC). The work of EWB, AS, AEJ, and KCR was supported by the Intramural Research programs of the National Institute on Drug Abuse. This material has been reviewed by the Walter Reed Army Institute of Research and the National Institute on Drug Abuse. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors and should not be construed as official or as reflecting true views of the Department of the Army, the Department of Defense, NIDA, NIH, or the US government. GRM, OBT, AS, KCR, AEJ, and EB are inventors of a provision patent application filed by the Henry M. Jackson Foundation for the Advancement of Military Medicine (provisional patent Serial No.: 62/960,187; January 13, 2020). The authors thank David McCurdy, Therese Oertel, Nadine Nehme, and Alexander Anderson for outstanding technical assistance.
Glossary
AbbreviationS
- ALF43A
Army Liposome Formulation with 43% cholesterol on aluminum hydroxide
- 6-AM
6-acetyl morphine
- 6-AmHap
N-((7S,7aR,12bS)-7-acetamido-3-methyl-2,3,4,4a,5,6,7,7a-octahydro-1H-4,12-methanobenzofuro [3,2-e]isoquinolin-9-yl)-3-(tritylthio)propanamide)
- BCA
bicinchoninic acid
- BSA
bovine serum albumin
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DMPG
1,2-dimyristoyl-sn-glycero-3-phosphoglycerol
- 3D-PHAD
monophosphoryl 3-Deacyl Lipid A
- ED
equilibrium dialysis
- ED50
50% effective dose
- ELISA
enzyme-linked immunosorbent assay
- IC50
50% inhibitory concentration
- IgG
immunoglobulin
- Kd
dissociation constant
- MALDI-TOF MS
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MPLA
monophosphoryl lipid A
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- LD50
50% lethal dose
- MPE
maximum potential effect
- MWCO
molecular weight cutoff
- para-AmFenHap
N-phenyl-N-(1-(4-(3-(tritylthio)propanamido)phenethyl)piperidin-4-yl)propionamide
- PBS
phosphate buffered saline
- s.c.
subcutaneous
- i.m.
intramuscular
- i.p.
intraperitoneal
- SM(PEG)2
succinimidyl-[(N-maleimidopropionamido)-diethylene glycol] ester
- TT
tetanus toxoid
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.bioconjchem.1c00179.
Supplemental methods, LC-MS/MS parameters, competition half-maximal inhibitory concentrations (IC50), serum binding to heroin and fentanyl mixture (PDF)
Author Present Address
⊥ Merck & Co., Inc., 126 E Lincoln Avenue, Rahway, New Jersey 07065, United States
Author Present Address
¶ Office of New Drug Products, Office of Pharmaceutical Quality, Center for Drug Evaluation and Research, Food and Drug Administration, 10903 New Hampshire Avenue, Silver Spring, Maryland 20993, United States
Author Present Address
# Pfizer, Inc., 401 N Middletown Rd, Pearl River, New York 10965, United States
The authors declare the following competing financial interest(s): GRM, OBT, AS, KCR, AEJ, and EB are inventors of a provision patent application filed by the Henry M. Jackson Foundation for the Advancement of Military Medicine (provisional patent Serial No.: 62/960,187; January 13, 2020).
Supplementary Material
References
- Socias M. E.; Wood E. (2017) Epidemic of deaths from fentanyl overdose. BMJ. 358, j4355. 10.1136/bmj.j4355. [DOI] [PubMed] [Google Scholar]
- Gostin L. O.; Hodge J. G. Jr.; Noe S. A. (2017) Reframing the Opioid Epidemic as a National Emergency. JAMA 318, 1539–1540. 10.1001/jama.2017.13358. [DOI] [PubMed] [Google Scholar]
- Ciccarone D. (2017) Fentanyl in the US heroin supply: A rapidly changing risk environment. Int. J. Drug Policy 46, 107–111. 10.1016/j.drugpo.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (2020). Overdose Death Rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates (accessed May 14, 2020).
- Frank R. G.; Pollack H. A. (2017) Addressing the Fentanyl Threat to Public Health. N. Engl. J. Med. 376, 605–607. 10.1056/NEJMp1615145. [DOI] [PubMed] [Google Scholar]
- Gable R. S. (2004) Comparison of acute lethal toxicity of commonly abused psychoactive substances. Addiction 99, 686–96. 10.1111/j.1360-0443.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (2020) Fentanyl drug profile. http://www.emcdda.europa.eu/publications/drug-profiles/fentanyl (accessed February 8, 2020).
- Solis E.; Cameron-Burr K. T.; Kiyatkin E. A. (2017) Heroin Contaminated with Fentanyl Dramatically Enhances Brain Hypoxia and Induces Brain Hypothermia. eNeuro 4, ENEURO.0323-17.2017. 10.1523/ENEURO.0323-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce P., Taylor J., Caulkins J., Kilmer B., Reuter P., and Stein B. (2019) The Future of Fentanyl and Other Synthetic Opioids, RAND Corporation, Santa Monica, CA. [Google Scholar]
- Pearson J.; Poklis J.; Poklis A.; Wolf C.; Mainland M.; Hair L.; Devers K.; Chrostowski L.; Arbefeville E.; Merves M. (2015) Postmortem Toxicology Findings of Acetyl Fentanyl, Fentanyl, and Morphine in Heroin Fatalities in Tampa, Florida. Acad. Forensic Pathol 5, 676–689. 10.23907/2015.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman R. A.; Morris N. P. (2020) Is It Time to Reschedule Heroin?. JAMA psychiatry. 77, 781. 10.1001/jamapsychiatry.2020.0607. [DOI] [PubMed] [Google Scholar]
- Dembek Z. F.; Chekol T.; Wu A. (2020) The Opioid Epidemic: Challenge to Military Medicine and National Security. Mil. Med. 185, e662–e667. 10.1093/milmed/usz487. [DOI] [PubMed] [Google Scholar]
- Caves J. P. J. (2019) in CSWMD Proceedings, pp 1–5, National Defense University Press. [Google Scholar]
- Talu A.; Rajaleid K.; Abel-Ollo K.; Ruutel K.; Rahu M.; Rhodes T.; Platt L.; Bobrova N.; Uuskula A. (2010) HIV infection and risk behaviour of primary fentanyl and amphetamine injectors in Tallinn, Estonia: implications for intervention. Int. J. Drug Policy 21, 56–63. 10.1016/j.drugpo.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Lambdin B. H.; Bluthenthal R. N.; Zibbell J. E.; Wenger L.; Simpson K.; Kral A. H. (2019) Associations between perceived illicit fentanyl use and infectious disease risks among people who inject drugs. Int. J. Drug Policy 74, 299–304. 10.1016/j.drugpo.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence C. S.; Zhou C.; Luo F.; Xu L. (2016) The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States, 2013. Med. Care 54, 901–6. 10.1097/MLR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C.; Volkow N. D. (2019) Management of opioid use disorder in the USA: present status and future directions. Lancet 393, 1760–1772. 10.1016/S0140-6736(18)33078-2. [DOI] [PubMed] [Google Scholar]
- Hoffman K. A.; Ponce Terashima J.; McCarty D. (2019) Opioid use disorder and treatment: challenges and opportunities. BMC Health Serv. Res. 19, 884. 10.1186/s12913-019-4751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton J. J.; Agnoli A. L.; Xing G.; Hang L.; Altan A. E.; Tancredi D. J.; Jerant A.; Magnan E. (2019) Trends and Rapidity of Dose Tapering Among Patients Prescribed Long-term Opioid Therapy, 2008–2017. JAMA Netw Open 2, e1916271. 10.1001/jamanetworkopen.2019.16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzasa Lynn R.; Galinkin J. L. (2018) Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther. Adv. Drug Saf. 9, 63–88. 10.1177/2042098617744161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. B.; Carlo D. J. (2019) Higher doses of naloxone are needed in the synthetic opioid era. Subs. Abuse Treat. Prev. Policy. 14, 6. 10.1186/s13011-019-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R.; Veliz S.; Singer D. (2020) Wooden chest syndrome: Beware of opioid antagonists, not just agonists. Am. J. Emerg. Med. 38, 411.e5–411.e6. 10.1016/j.ajem.2019.09.009. [DOI] [PubMed] [Google Scholar]
- Volkow N. D.; Collins F. S. (2017) The Role of Science in Addressing the Opioid Crisis. N. Engl. J. Med. 377, 391–394. 10.1056/NEJMsr1706626. [DOI] [PubMed] [Google Scholar]
- Olson M. E.; Janda K. D. (2018) Vaccines to combat the opioid crisis: Vaccines that prevent opioids and other substances of abuse from entering the brain could effectively treat addiction and abuse. EMBO Rep. 19, 5–9. 10.15252/embr.201745322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer P. T.; Janda K. D. (2017) Conjugate Vaccine Immunotherapy for Substance Use Disorder. Pharmacol. Rev. 69, 298–315. 10.1124/pr.117.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr C.; Pravetoni M. (2019) Vaccines to treat opioid use disorders and to reduce opioid overdoses. Neuropsychopharmacology 44, 217–218. 10.1038/s41386-018-0197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen J. D.; Bremer P. T.; Hwang C. S.; Vandewater S. A.; Collins K. C.; Creehan K. M.; Janda K. D.; Taffe M. A. (2017) Effective active vaccination against methamphetamine in female rats. Drug Alcohol Depend. 175, 179–186. 10.1016/j.drugalcdep.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey B. M.; Kosten T. R.; Orson F. M. (2010) Anti-cocaine vaccine development. Expert Rev. Vaccines 9, 1109–1114. 10.1586/erv.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh M. D.; Peterson S. J.; Laudenbach M.; Baruffaldi F.; Carroll F. I.; Comer S. D.; Navarro H. A.; Langston T. L.; Runyon S. P.; Winston S.; et al. (2017) Safety and efficacy of an oxycodone vaccine: Addressing some of the unique considerations posed by opioid abuse. PLoS One 12, e0184876. 10.1371/journal.pone.0184876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulima A.; Jalah R.; Antoline J. F. G.; Torres O. B.; Imler G. H.; Deschamps J. R.; Beck Z.; Alving C. R.; Jacobson A. E.; Rice K. C.; et al. (2018) A Stable Heroin Analogue That Can Serve as a Vaccine Hapten to Induce Antibodies That Block the Effects of Heroin and Its Metabolites in Rodents and That Cross-React Immunologically with Related Drugs of Abuse. J. Med. Chem. 61, 329–343. 10.1021/acs.jmedchem.7b01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C. S.; Smith L. C.; Natori Y.; Ellis B.; Zhou B.; Janda K. D. (2018) Efficacious Vaccine against Heroin Contaminated with Fentanyl. ACS Chem. Neurosci. 9, 1269–1275. 10.1021/acschemneuro.8b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend E. A.; Blake S.; Faunce K. E.; Hwang C. S.; Natori Y.; Zhou B.; Bremer P. T.; Janda K. D.; Banks M. L. (2019) Conjugate vaccine produces long-lasting attenuation of fentanyl vs. food choice and blocks expression of opioid withdrawal-induced increases in fentanyl choice in rats. Neuropsychopharmacology 44, 1681–1689. 10.1038/s41386-019-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney R. D.; Blake S.; Bremer P. T.; Zhou B.; Hwang C. S.; Poklis J. L.; Janda K. D.; Banks M. L. (2019) Vaccine blunts fentanyl potency in male rhesus monkeys. Neuropharmacology 158, 107730. 10.1016/j.neuropharm.2019.107730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh M. D.; Baruffaldi F.; Peterson S. J.; Le Naour M.; Harmon T. M.; Vigliaturo J. R.; Pentel P. R.; Pravetoni M. (2019) A Fentanyl Vaccine Alters Fentanyl Distribution and Protects against Fentanyl-Induced Effects in Mice and Rats. J. Pharmacol. Exp. Ther. 368, 282–291. 10.1124/jpet.118.253674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer P. T.; Kimishima A.; Schlosburg J. E.; Zhou B.; Collins K. C.; Janda K. D. (2016) Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew. Chem., Int. Ed. 55, 3772–3775. 10.1002/anie.201511654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend E. A.; Bremer P. T.; Faunce K. E.; Negus S. S.; Jaster A. M.; Robinson H. L.; Janda K. D.; Banks M. L. (2020) Evaluation of a Dual Fentanyl/Heroin Vaccine on the anti-nociceptive and Reinforcing Effects of a Fentanyl/Heroin Mixture in Male and Female Rats. ACS Chem. Neurosci. 11, 1300. 10.1021/acschemneuro.0c00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubanks L. M.; Blake S.; Natori Y.; Ellis B.; Bremer P. T.; Janda K. D. (2021) A Highly Efficacious Carfentanil Vaccine That Blunts Opioid-Induced anti-nociception and Respiratory Depression. ACS Chem. Biol. 16, 277. 10.1021/acschembio.1c00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres O. B., Alving C. R., Jacobson A. E., Rice K. C., and Matyas G. R. (2016) Practical Considerations for the Development of Vaccines Against Drugs of Abuse, in Biologics to Treat Substance Use Disorders: Vaccines, Monoclonal Antibodies, and Enzymes (Montoya I. D., Ed.) pp 397–424, Springer International Publishing, Cham. [Google Scholar]
- Schlingmann B.; Castiglia K. R.; Stobart C. C.; Moore M. L. (2018) Polyvalent vaccines: High-maintenance heroes. PLoS Pathog. 14, e1006904–e1006904. 10.1371/journal.ppat.1006904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M.; Raleigh M. D.; Le Naour M.; Tucker A. M.; Harmon T. M.; Jones J. M.; Birnbaum A. K.; Portoghese P. S.; Pentel P. R. (2012) Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine 30, 4617–24. 10.1016/j.vaccine.2012.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres O. B.; Matyas G. R.; Rao M.; Peachman K. K.; Jalah R.; Beck Z.; Michael N. L.; Rice K. C.; Jacobson A. E.; Alving C. R. (2017) Heroin-HIV-1 (H2) vaccine: induction of dual immunologic effects with a heroin hapten-conjugate and an HIV-1 envelope V2 peptide with liposomal lipid A as an adjuvant. npj Vaccines 2, 13. 10.1038/s41541-017-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C. S.; Smith L. C.; Natori Y.; Ellis B.; Zhou B.; Janda K. D. (2018) Improved Admixture Vaccine of Fentanyl and Heroin Hapten Immunoconjugates: anti-nociceptive Evaluation of Fentanyl-Contaminated Heroin. ACS omega 3, 11537–11543. 10.1021/acsomega.8b01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natori Y.; Hwang C. S.; Lin L.; Smith L. C.; Zhou B.; Janda K. D. (2019) A chemically contiguous hapten approach for a heroin-fentanyl vaccine. Beilstein J. Org. Chem. 15, 1020–1031. 10.3762/bjoc.15.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R. C.; Bow E. W.; Whalen C.; Torres O. B.; Sulima A.; Beck Z.; Jacobson A. E.; Rice K. C.; Matyas G. R. (2020) Novel vaccine that blunts fentanyl effects and sequesters ultrapotent fentanyl analogs. Mol. Pharmaceutics 17, 3447–3460. 10.1021/acs.molpharmaceut.0c00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alving C. R.; Peachman K. K.; Matyas G. R.; Rao M.; Beck Z. (2020) Army Liposome Formulation (ALF) family of vaccine adjuvants. Expert Rev. Vaccines 19, 1–14. 10.1080/14760584.2020.1745636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres O. B.; Antoline J. F.; Li F.; Jalah R.; Jacobson A. E.; Rice K. C.; Alving C. R.; Matyas G. R. (2016) A simple nonradioactive method for the determination of the binding affinities of antibodies induced by hapten bioconjugates for drugs of abuse. Anal. Bioanal. Chem. 408, 1191–204. 10.1007/s00216-015-9223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K. E.; de Villiers S. H.; Pravetoni M.; Pentel P. R. (2013) Immunogenicity of individual vaccine components in a bivalent nicotine vaccine differ according to vaccine formulation and administration conditions. PLoS One 8, e82557. 10.1371/journal.pone.0082557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Bungener L.; ter Veer W.; Coller B.-A.; Wilschut J.; Huckriede A. (2011) Preclinical evaluation of the saponin derivative GPI-0100 as an immunostimulating and dose-sparing adjuvant for pandemic influenza vaccines. Vaccine 29, 2037–2043. 10.1016/j.vaccine.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Boyle J.; Eastman D.; Millar C.; Camuglia S.; Cox J.; Pearse M.; Good J.; Drane D. (2007) The utility of ISCOMATRIX adjuvant for dose reduction of antigen for vaccines requiring antibody responses. Vaccine 25, 2541–2544. 10.1016/j.vaccine.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Wittrup D., Tidor B., Hackel B., and Sarkar C. (2019) Quantitative fundamentals of molecular and cellular bioengineering, The MIT Press, Cambridge, MA. [Google Scholar]
- Jalah R.; Torres O. B.; Mayorov A. V.; Li F.; Antoline J. F.; Jacobson A. E.; Rice K. C.; Deschamps J. R.; Beck Z.; Alving C. R.; et al. (2015) Efficacy, but not antibody titer or affinity, of a heroin hapten conjugate vaccine correlates with increasing hapten densities on tetanus toxoid, but not on CRM197 carriers. Bioconjugate Chem. 26, 1041–53. 10.1021/acs.bioconjchem.5b00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo E. F.; Stinus L.; Cador M.; Mir D. (1994) Effects of morphine and naloxone on behaviour in the hotplate test: an ethopharmacological study in the rat. Psychopharmacology (Berl.) 113, 500–10. 10.1007/BF02245230. [DOI] [PubMed] [Google Scholar]
- Plone M. A.; Emerich D. F.; Lindner M. D. (1996) Individual differences in the hotplate test and effects of habituation on sensitivity to morphine. Pain 66, 265–70. 10.1016/0304-3959(96)03048-5. [DOI] [PubMed] [Google Scholar]
- Coffman R. L.; Sher A.; Seder R. A. (2010) Vaccine adjuvants: putting innate immunity to work. Immunity 33, 492–503. 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (2018) TDVAX. https://www.fda.gov/vaccines-blood-biologics/vaccines/tdvax (accessed February 8, 2020).
- Alving C. R.; Beck Z.; Matyas G. R.; Rao M. (2016) Liposomal adjuvants for human vaccines. Expert Opin. Drug Delivery 13, 807–16. 10.1517/17425247.2016.1151871. [DOI] [PubMed] [Google Scholar]
- Torres O. B.; Jalah R.; Rice K. C.; Li F.; Antoline J. F.; Iyer M. R.; Jacobson A. E.; Boutaghou M. N.; Alving C. R.; Matyas G. R. (2014) Characterization and optimization of heroin hapten-BSA conjugates: method development for the synthesis of reproducible hapten-based vaccines. Anal. Bioanal. Chem. 406, 5927–37. 10.1007/s00216-014-8035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres O. B.; Duval A. J.; Sulima A.; Antoline J. F. G.; Jacobson A. E.; Rice K. C.; Alving C. R.; Matyas G. R. (2018) A rapid solution-based method for determining the affinity of heroin hapten-induced antibodies to heroin, its metabolites, and other opioids. Anal. Bioanal. Chem. 410, 3885–3903. 10.1007/s00216-018-1060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas G. R.; Mayorov A. V.; Rice K. C.; Jacobson A. E.; Cheng K.; Iyer M. R.; Li F.; Beck Z.; Janda K. D.; Alving C. R. (2013) Liposomes containing monophosphoryl lipid A: a potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine 31, 2804–10. 10.1016/j.vaccine.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck Z.; Torres O. B.; Matyas G. R.; Lanar D. E.; Alving C. R. (2018) Immune response to antigen adsorbed to aluminum hydroxide particles: Effects of co-adsorption of ALF or ALFQ adjuvant to the aluminum-antigen complex. J. Controlled Release 275, 12–19. 10.1016/j.jconrel.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals, National Academies Press (US), National Academy of Sciences, Washington, DC, 2011. [Google Scholar]
- Bannon A. W.; Malmberg A. B. (2007) Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr. Protoc. Neurosci. 1. 10.1002/0471142301.ns0809s41. [DOI] [PubMed] [Google Scholar]
- Jones J. M.; Raleigh M. D.; Pentel P. R.; Harmon T. M.; Keyler D. E.; Remmel R. P.; Birnbaum A. K. (2013) Stability of heroin, 6-monoacetylmorphine, and morphine in biological samples and validation of an LC-MS assay for delayed analyses of pharmacokinetic samples in rats. J. Pharm. Biomed. Anal. 74, 291–7. 10.1016/j.jpba.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H., and Christopoulos A. (2003) Fitting models to biological data using linear and nonlinear regression, GraphPad Software Inc., San Diego, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.