Abstract
Fatigue symptoms are very common among persons living with HIV (PLWH). Fatigue is related to functional and psychological problems and to treatment nonadherence. Using secondary data from ecological momentary assessment, we examined fatigue as a predictor of PLWH everyday experiences. In bidirectional analyses based on the shape shifters model, we also examined these experiences as predictors of fatigue. Data were examined from 67 PLWH who completed daily surveys on a handheld computer daily. Brief validated scales were used to assess participants’ control beliefs, mood, stress, coping, social support, experience of stigma, and motivation. At the beginning and end of the study, fatigue was measured with two CES-D items that have been used in past HIV symptom research. Multilevel models and logistic regression were used to test reciprocal predictive relationships between variables. Moderate to severe fatigue affected 45% of PLWH in the study. Initial fatigue predicted PLWH subsequent overall level of control beliefs, mood, stress, coping, and social support, all p < .05. These state variables remained relatively constant over time, regardless of participants’ initial fatigue. In tests for reciprocal relationships with 33 PLWH, average daily stress, OR = 4.74, and stigma, OR = 4.86, also predicted later fatigue. Fatigue predicted several daily survey variables including stress and social support. Stress and support in turn predicted fatigue at a later time, suggesting a self-perpetuating cycle but also a possible avenue for intervention. Future studies should examine daily variation in fatigue among PLWH and its relation to other everyday experiences and behaviors.
Keywords: adherence, coping, ecological momentary assessment, fatigue, HIV
Fatigue is the most common symptom reported by persons living with HIV (PLWH: Erlandson, Schrack, Jankowski, Brown, & Campbell, 2014), occurring as part of a symptom cluster with sleep problems, depression, and cognitive slowing (Sousa, Kwok, & Tann, 2004). Although prevalence estimates vary across studies, anywhere from 20% to 60% of PLWH will experience clinically significant levels of fatigue (Barroso & Voss, 2013; Jong et al., 2010).
PLWH often report fatigue as a side effect of antiretroviral treatment (ART), and a recent meta-analysis also identified fatigue as a significant risk factor for ART nonadherence (Al-Dakkak et al., 2013). For example, in a South African study, 19.6% of PLWH said they experienced ART-related fatigue, and nearly half of those with fatigue said they were non-adherent because of it (Bhat et al., 2010). Some potential reasons for this association are distress about fatigue, low energy leading to functional limitations, or sleeping through a scheduled dose time (Bhat et al., 2010; Gay et al., 2011).
Fatigue may also have an etiology in HIV disease itself. More than other symptoms, fatigue may be a marker for HIV disease progression (Cook, Sousa, Matthews, Meek, & Kwong, 2011). Although biomarkers related to fatigue in HIV have been difficult to isolate (Barroso & Voss, 2013), in some studies PLWH fatigue appears connected to inflammatory processes like cytokine activation (Deeks, Tracy, & Douek, 2013; Foster et al., 2012) as is seen in other chronic diseases (Kim, Barsevick, Fang, & Miaskowski, 2012). More clearly, fatigue is linked to reduced activity and impaired sleep among PLWH, as well as depressed mood and cognitive confusion (Barroso & Voss, 2013). Other potential correlates of fatigue in PLWH include accelerated or accentuated aging processes (Pathai, Bajillan, Landay, & High, 2014), comorbid chronic illnesses, and pain or disability, all of which are common in this population (Nichols et al., 2002).
Shape Shifters Model of Symptom Science
The University of Colorado’s biobehavioral shape shifters framework (Corwin, Meek, Cook, Lowe, & Sousa, 2012) suggests that symptoms are integrated biobehavioral constructs that can be meaningfully discussed as physiological or psychological but are not primarily one or the other; they are both. They can be measured in multiple ways without presupposing that mental states cause physical ones or vice-versa. The model further suggests that symptoms can have bidirectional relationships with other physiological, psychological, and behavioral variables, both influencing them and being influenced by them. Most importantly, the shape shifters model suggests that these bidirectional relationships can occur for the same symptom in the same individual over time, taking the form of either a self-correcting homeostatic cycle in which symptoms lead to physiological, psychological, or behavioral sequelae that reduce the symptom via a feedback loop; or a self-perpetuating cycle in which symptoms lead to physiological, psychological, or behavioral sequelae that in turn further exacerbate the symptom.
Either type of relationship seems possible in the case of fatigue and daily experiences of PLWH. For example, fatigue might limit coping or increase stress in ways that exacerbate fatigue; or else fatigue might be self-correcting if behavioral changes in response to the symptom make it less intense. This type of model is ideally tested using statistical methods that account for time-dependent relationships, such as multilevel modeling (Walls, Jung, & Schwartz, 2006) or latent class analysis (Schmiege, Meek, Bryan, & Petersen, 2012).
Accounting for reciprocal relationships between variables over time requires that data be collected from individuals more frequently or for a longer period of time than has been typical, even in longitudinal research (Hamaker, 2012). Fortunately, new technology allows researchers to collect intensive longitudinal data, as reflected in recent methodological innovations such as ecological momentary assessment (Walls & Schafer, 2006) or self-monitoring technologies that contribute to the “quantified self” (Swan, 2013). Data collected using these methods are particularly appropriate for tests of the shape shifters model.
Purpose of the Study
This study was a secondary analysis of data from a research project designed to test seven different types of everyday experiences as predictors of medication adherence in HIV. Based on the shape shifters framework, our goals in this study as depicted in Figure 1 were to test the impact of baseline fatigue on PLWH’s everyday experiences, and also to examine predictive relationships in the opposite direction, in which everyday experiences in turn might influence fatigue.
FIGURE 1.
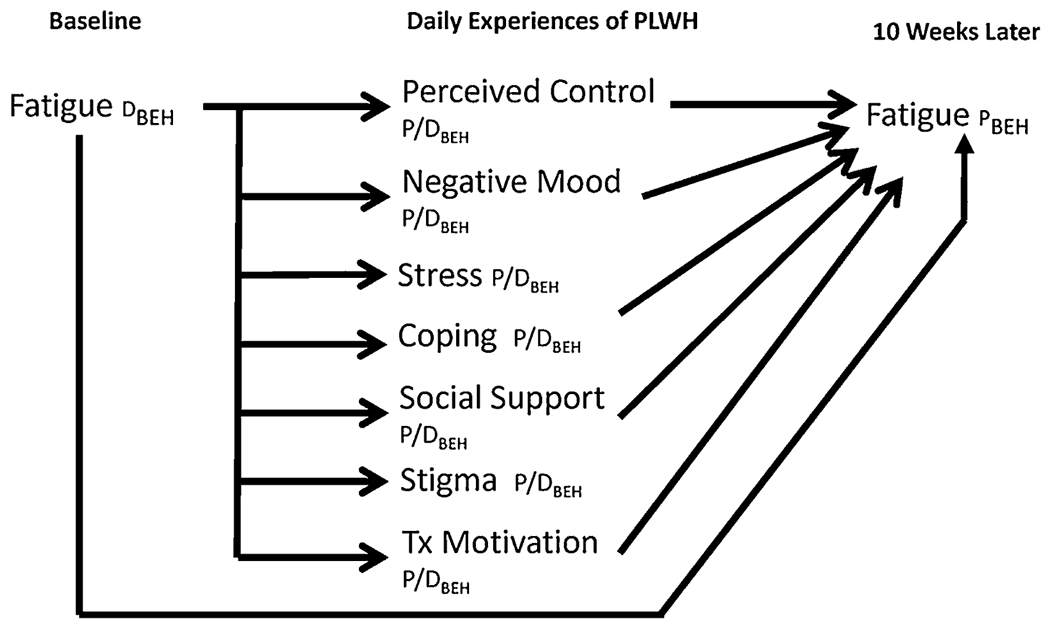
Shape shifter diagram showing hypothesized bidirectional relationships. PLWH=persons living with HIV. Tx=antiretroviral treatment. Other notation comes from the “shape shifters” model, in which D=“determinant,” a variable that predicts a biobehavioral phenomenon; P=“phenomenon,” a variable that is predicted by determinants; P/D=a variable that serves as both a phenomenon and a determinant in the overall process; and BEH=a behavioral measure. In the shape shifters model, phenomena and determinants are understood to be intrinsically biobehavioral, so “BEH” (or “BIO” in other diagrams) is used to denote the measurement method only, and not a construct’s essential nature.
Methods
Overview of Design
Daily experiences of PLWH were measured via an electronic survey completed on a smartphone once per day for 10 weeks. The survey was completed at a randomly selected time, when cued by an alarm on the phone. The survey included measures of seven momentary state variables that have been previously studied in PLWH (Cook, McElwain, & Bradley-Springer, 2010): perceived control over daily life, negative mood, acute and chronic stress, coping strategies used, perceived social support, HIV-related stigma, and motivation for ART.
We tested initial fatigue as a predictor of each of these daily measures in a longitudinal design. To examine reciprocal relationships as suggested by the shape shifters model, we then tested participants’ average score on each of the daily measures as a prospective predictor of a second fatigue measure at the end of the daily monitoring period. Because we had data on fatigue both before and after the 10-week daily monitoring period, we were able to examine prospective relationships between determinant and outcome variables in both directions.
Although specific causal conclusions could not be made with this design, evidence about the relationship between fatigue symptoms and everyday experiences based on intensive longitudinal data would be stronger than simple correlations because it would include the element of temporal precedence. Correlation is the first of Hume’s 3 criteria for causation (Hume, 1748/1993), and a temporal sequence is the second.
Participants
Participants were 19 women and 68 men with HIV recruited from an outpatient infectious disease clinic in Denver, Colorado. The Colorado Multiple Institutional Review Board approved the study, and all participants provided written informed consent. PLWH were recruited at regularly scheduled clinic visits with their usual HIV care providers, who briefly introduced the procedures and then referred interested patients to a research team member to complete the informed consent process.
Study inclusion criteria were: (a) documented HIV infection and current ART based on medical records; (b) ability to speak, read, and write English as either a first or second language; (c) older than 18 years of age and younger than 81 years of age; and (d) demonstrated ability to use a smartphone after initial training. Exclusion criteria were any level of current substance abuse, cognitive impairment, major psychiatric disorder, comorbid medical disorder, or other condition that would, in the judgment of the referring clinician, interfere with study participation.
Of 158 PLWH approached about the study, 87 were eligible and chose to participate. There were only seven exclusions, all of which were for either serious physical illness (e.g., hepatitis C or kidney failure) or mental illness (e.g., bipolar disorder or schizophrenia). Another 17 potential participants did not meet inclusion criteria because they did not manage their own medication, did not speak English, or were not currently receiving medication. Finally, 47 patients were eligible but declined to participate. There were no exclusions related to use of the daily survey technology.
Participants’ average age was M=40.0 years (SD=8.84, range: 21–59). About half of participants (41/87) were homosexual men, with three women also reporting a same-sex orientation and the remaining participants identifying themselves as heterosexual. Participants were diverse in terms of race and ethnicity (49% White non-Hispanic, 23% Latino/Latina, 15% African-American, 2% Native American, 7% multi-racial, and 3% other or unspecified). Participants’ average level of education was 13.4 years (SD=2.30), and 79 of 87 participants had at least a high school diploma. Fully 25% of participants were homeless or unstably housed, with another 9% residing in supported living arrangements. Most participants were either Medicare or Medicaid recipients (41%) or receiving health care through Ryan White program funding (38%). In general, these demographic characteristics suggest that the participants were representative of the diverse, multi-problem population affected by the United States HIV epidemic.
Clinically, participants’ HIV was generally well-managed, and 91.1% had undetectable viral loads (VL < 200). The median CD-4 level was 494 (IQR=677). The average number of ART doses per day was 1.45, with slightly more than half of participants on a once-daily regimen. Most participants had multiple comorbid conditions, including 54% with a comorbid substance abuse diagnosis and 25% with a serious and persistent mental illness.
Procedure
Baseline and follow-up data collection.
During an intake session, each participant completed a packet of baseline self-report questionnaires. The intake session was held in the same clinic where the participant received primary HIV care, and was usually completed immediately after enrollment but in a few cases was scheduled at a later time. All measures were administered by a professional research assistant with extensive prior experience and training on data collection methods and HIPAA regulations.
Participants then received a smartphone for daily survey data collection. After 10 weeks, participants returned to the clinic for a follow-up session with the same research assistant, and completed an identical packet of self-report measures to assess the same variables at the end of the study. Participants were paid $25 each for completing the intake and follow-up sessions.
Daily surveys.
During the intake session, the research assistant provided the participant with a smartphone (Samsung hardware and Android operating system) that was pre-loaded with Apptive® scheduling software. This software delivered a cue for the participant to take an online survey at a randomly selected time each day. Participants completed the survey by clicking an URL to reach a secure questionnaire hosted by SurveyMonkey®. During the intake session participants were instructed on how to use the smartphone and practiced completing the daily survey once with the research assistant. Participants were allowed to keep their smartphones at the end of the study if they completed at least 1 month of surveys, but the study paid for telephone service fees only during the data collection period.
Measures
Demographic and clinical data.
Participants provided demographic information on their age, gender, sexual orientation, level of education, race/ethnicity, housing or homelessness, and insurance or other access to care. Other data were extracted from participants’ medical records with authorization, including their latest HIV viral load, latest CD4+ T cell lab result, ART medication regimen, comorbid medical conditions, mental health conditions, and substance use. The intake packet also included baseline measures of self-efficacy, stress, coping, social support, HIV-related stigma, and ART knowledge (data not presented here).
Items measuring fatigue.
The intake packet included a widely used and well-validated measure of depressive symptoms, the Center for Epidemiological Studies Depression scale (CES-D: Radloff, 1977), that includes a subscale on somatic complaints (Hertzog, Van Alstine, Usala, Hultsch, & Dixon, 1990). The entire CES-D is strongly related to frailty and fatigue among older adults (Berkman et al., 1986).
Two CES-D items in particular have been used to study fatigue—sometimes also called “exhaustion”—in the context of HIV (Erlandson et al., 2012a,b, 2013; Kooij et al., 2016): CES-D item #7 reads “I felt that everything I did was an effort” and item #20 reads “I could not get going.” For clarity we refer to these two aspects of fatigue as effort (CES-D item 7) and inertia (CES-D item 20). Participants were asked to rate each CES-D item on a 4-point Likert-type scale ranging from 0 = rarely (less than 1 Dayin the last week) to 3 = most or all of the time (5–7 days in the last week).
These two items were correlated only r=.32 in the current study and may measure separate aspects of PLWH’s fatigue symptom experience; when we attempted to combine them into a scale it had low internal consistency reliability, α=.49. We therefore analyzed each of the fatigue items separately because they appeared to convey different information.
Daily smartphone surveys.
Most survey items were designed specifically for daily data collection and were tested in prior daily survey research with PLWH (Cook et al., 2010). Items offered response choices on a 4-point Likert-type scale from the Diary of Ambulatory Behavioral States (DABS: Kamarck, 1998): 1=NO!!, 2=no??, 3=yes??, or 4=YES!!
Control beliefs were measured with 3 DABS items plus a decisional balance question with higher scores indicating greater perceived control; the combined subscale was internally consistent in prior research (Cook et al., 2010), α = .84, and DABS items have shown concurrent and predictive validity.
Mood was measured on a continuum with three items from the DABS on which higher scores indicated better mood, with α=.93 and prior evidence for concurrent and predictive validity (Cook et al., 2010).
Stress was measured with six items adapted from the Daily Hassles Scale in which higher scores reflected more stress from various sources, with α=.67 in the current study and prior evidence for construct and divergent validity (Holm & Holroyd, 1992).
Coping was measured with the 10-item Assessment of Daily Coping, on which higher scores meant more intensive coping efforts. This instrument has well-established construct validity in prior research (Stone & Neale, 1984) and had internal consistency reliability of α=.86 in the current study.
Social support was measured with two items from another DABS subscale with prior reliability and validity evidence, α=.95 (Cook et al., 2010), on which higher scores indicated more perceived support.
HIV-related stigma was measured with two items adapted from the HIV Stigma Scale (Berger, Ferrans, & Lashley, 2001) with higher scores indicating more perceived stigma, which had internal consistency reliability of α = .65 in a prior study (Cook et al., 2010). Stigma had a very low correlation with social support and was therefore considered separately.
Finally, motivation for ART was measured using a scale adapted from Herzog and Blagg (2007), with items such as “having a plan to take medication,” “intending to take medication,” and “having the desire to take medication.” This scale, on which higher scores indicated more motivation, had internal consistency reliability of α=.79 and has shown predictive validity greater than that of the widely used stages-of-change measure (Herzog & Blagg, 2007).
Data Analysis
Preliminary data analysis.
Baseline demographic and clinical data were analyzed descriptively to characterize the sample. Participants completed a mean of 39 surveys over the 10 weeks (range: 1–114), or 3,254 total from 87 participants. A subset of 20 participants who completed very few daily surveys (≤10) were removed from analyses due to concerns about the accuracy of multilevel estimates in cases with few data points, resulting in 67 participants with an average of 47 surveys each (3,177 total surveys), reflecting survey completion on 68% of possible days. As described below, we used a multilevel modeling framework for analysis that includes all available data points and allows for different numbers of observations per participant.
Within-person effects.
Analyses were conducted in two stages, corresponding to the two possible directions of effects predicted by the shape shifters symptom model. In the first stage, we used MLM to test fatigue as a predictor of daily experiences. MLM handles multiple observations per participant over time by generating a unique “level 1” linear model for each participant with its own slope and intercept. Those slopes and intercepts are then treated as dependent variables in “level 2” equations with predictor variables that are constant at the person level, such as baseline fatigue. MLM has many advantages over traditional analysis strategies for within-person data such as repeated-measures ANOVA: It can accommodate differing numbers of data points per person at different time intervals, it compensates for missing data via maximum likelihood estimation, and it allows for a combination of level 1 (within-person) and level 2 (between-person) predictors as well as for tests of interactions between them. The inclusion of time as a within-person predictor allowed for exploratory tests of moderator effects in addition to simple linear relationships.
The following equations were used to estimate effects of baseline fatigue on average levels of the daily survey variables (β0) and on the trajectory of change in each of the daily survey variables over time (β1).
A significant effect of γ10 indicates a main effect of fatigue on that variable, while a significant effect of γ11 indicates a moderating effect in which the participant’s baseline fatigue predicts the magnitude of change in that variable over time, with positive effects showing acceleration and negative effects showing deceleration in the average rate of change.
Between-person effects.
In the second stage of analysis, we tested the average level of each daily survey variable over the 10-week monitoring period as a predictor of that participant’s fatigue level at the end of the monitoring period. This analysis used scores on the second (follow-up) fatigue questionnaire as the criterion variable. Analyses for this stage were completed using ordinal logistic regression.
Power analysis.
Power for MLM depends on the number of observations, not the number of participants, after correction for the intra-class correlation (ICC) of observations from the same person, which we conservatively estimated at .70 (Hox, 2002). With as few as 33 data points (consistent with data completeness in a pilot study: Cook et al., 2010) from as few as 62 participants, power was .80 to detect moderate effects of r>.39 at α = .05 using MLM. A priori power was not calculated for the analyses of reciprocal effects of daily experiences on fatigue, but post hoc analysis showed that these tests using logistic regression also had power =.80 to detect moderate effects of φ>.37 at α = .05 based on the final sample size of 33 PLWH at the last observation point.
Results
Representativeness of the Analytic Sample
All 87 participants had baseline data on fatigue, 67 had sufficient daily survey data for analysis, and 33 completed a second fatigue measure at the end of the study. The 67 participants analyzed in the test of fatigue as a predictor of daily states were similar to the other 20 PLWH with missing data in terms of age, gender, race/ethnicity, sexual orientation, and years of education (all p>.11). Similarly, there were no differences between the 33 PLWH included in the analysis of follow-up fatigue data and the participants with missing data in terms of gender, race/ethnicity, sexual orientation, or years of education (all p>.12). There was a slight difference in age between those lost to follow-up at this point and those included in the final analytic sample, t(78)=2.07, p=.04, with those included in the analysis slightly older on average, M=44 years (SD=8.50), than those lost to follow-up, M=40 years (SD=8.80). This minor difference in age was not considered clinically significant with respect to participants’ experience of fatigue symptoms.
Prevalence and Severity of Fatigue
Patients’ self-reported level of fatigue was classified as none, mild, moderate, or severe based on their response choices on the 2 CES-D fatigue items. Based on the symptom of inertia, 44% of PLWH reported no fatigue, 24% reported mild fatigue, 16% reported moderate fatigue, and 16% reported severe fatigue. Based on effort, only 30% of PLWH reported no fatigue, with the others reporting fatigue that was was 28% mild, 17% moderate, and 25% severe. In total, 45% of PLWH reported fatigue in either the moderate or severe categories on at least one of the two fatigue items.
For both CES-D items, fatigue at the baseline assessment predicted fatigue at the time of the 10-week follow-up assessment. Both correlations were moderate to strong, r=.61, p=.006 for effort, and r=.65, p<.001 for inertia. It was not possible to formally test the effect of time on fatigue in a multilevel framework because there were only two time points for each of the fatigue items. However, similar mean scores at both time points suggest that participants’ level of fatigue stayed relatively constant over the 10 weeks of the study.
Forward Prediction of Everyday Experiences From Fatigue
There was no evidence of fatigue as a moderator of changes in participants’ scores over time on any of the daily survey scales—i.e., no significant time-by-fatigue interactions as represented by the γ11 coefficient. There was evidence for main effects of fatigue; however, where baseline levels of fatigue predicted average levels on several of the daily survey scales as measured over time. These effects are represented by the γ10 coefficient in the equations above, and are shown in Table 1. Specifically, higher fatigue as measured by both effort and inertia predicted worse mood and greater stress. Additionally, higher fatigue as measured by inertia predicted decreased control beliefs, decreased coping, and decreased perceptions of social support. There were no effects of either fatigue item on participants’ experience of HIV-related stigma or their motivation for ART.
Table 1.
Everyday Experiences Predicted by Initial Fatigue (N=67)
| Measures of Daily Experiences | Effect of Effort Item |
Effect of Inertia Item |
||
|---|---|---|---|---|
| Statistic | p | Statistic | p | |
| Control beliefs | b (SE)=−.11 (.06) | .06 | b (SE)=−.18 (.05) | .001*** |
| t (df 61.4)=−1.93 | t (df 61.7)=−3.46 | |||
| Mood | b (SE)=−.17 (.08) | .04* | b (SE)=−.23 (.08) | .004** |
| t (df 61.4)=−2.15 | t (df 61.6)=−2.99 | |||
| Stress | b (SE)=.14 (.06) | .03* | b (SE)=.14 (.06) | .02* |
| t (df 48.4)=2.18 | t (df 47.3)=2.35 | |||
| Coping | b (SE)=−.002 (.06) | .98 | b (SE)=−.14 (.06) | .03* |
| t (df 57.2)=−.02 | t (df 56.6)=−2.31 | |||
| Social support | b (SE)=−.13 (.08) | .10 | b (SE)=−.23 (.07) | .003** |
| t (df 61.8)=−1.68, p= | t (df 62.2)=−3.09 | |||
| HIV-related stigma | b (SE)=.08 (.07) | .25 | b (SE)=.10 (.06) | .12 |
| t (df 61.7)=1.17 | t (df 61.6)=1.60 | |||
| Motivation for ART | b (SE)=−.05 (.04) | .21 | b (SE)=−.03 (.04) | .41 |
| t (df 59.9)=−1.27 | t (df 60.1)=−.83 | |||
Notes. SE, standard error; ff, degrees of freedom. Analyses were conducted in a multilevel modeling (MLM) framework to address outcomes measured over several weeks per person. Standardized betas are given for tests conducted using MLM. CES-D scores were grand-mean-centered. Degrees of freedom were calculated based on a Satterthwaite approximation.
p≤.05.
p≤.01.
p≤.001.
Reciprocal Prediction of Fatigue From Everyday Experiences
As shown in Table 2, the average level of each daily survey was unrelated to effort as an indicator of fatigue at followup. In contrast, higher average stress (OR=4.74, p=.02), greater social support (OR=.39, p=.05), and greater reported HIV-related stigma (OR=4.86, p=.01) predicted more inertia fatigue symptoms at follow-up. There were no effects of the other four daily experience variables on fatigue. Figure 2 presents a revised shape shifter diagram showing only those relationships between variables that were statistically significant.
Table 2.
Fatigue Predicted by Everyday Experiences (n=33)
| Averaged Daily Experience Measures | Prediction of Effort Item |
Prediction of Inertia Item |
||||
|---|---|---|---|---|---|---|
| OR | CI | p | OR | CI | p | |
| Control beliefs | .74 | (.21, 2.65) | .65 | .41 | (.11, 1.48) | .17 |
| Mood | .63 | (.26, 1.52) | .30 | .43 | (.17, 1.08) | .07 |
| Stress | 2.84 | (.82, 9.86) | .10 | 4.74 | (1.30, 17.22) | .02* |
| Coping | 1.06 | (.31, 3.66) | .93 | .59 | (.17, 2.06) | .41 |
| Social support | .80 | (.33, 1.95) | .62 | .39 | (.15, 1.01) | .05* |
| HIV-related stigma | 2.02 | (.70, 5.79) | .19 | 4.86 | (1.51, 15.57) | .01** |
| Motivation for ART | 3.62 | (.74, 17.65) | .11 | 2.85 | (.63, 12.81) | .17 |
Notes. OR, odds ratio; CI, confidence interval. Analyses were conducted as bivariate ordinal logistic regression models to predict posttest CES-D effort and inertia scores from each of the everyday experience variables (averaged over study days). 95% confidence intervals around the odds ratios are presented.
p≤.05.
p≤.01.
FIGURE 2.
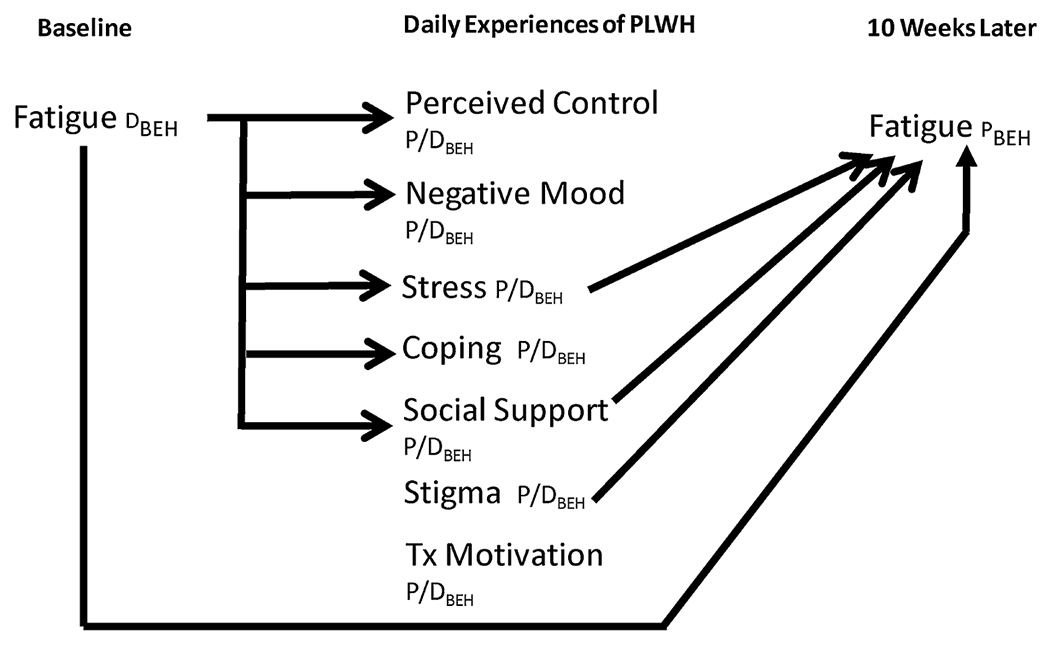
Revised shape shifter diagram showing actual relationships. Tx=antiretroviral treatment; PLWH=persons living with HIV; D=“determinant,” a variable that predicts a biobehavioral phenomenon; P=“phenomenon,” a variable that is predicted by determinants; P/D=a variable that serves as both a phenomenon and a determinant in the overall process; BEH=behavioral measure of a variable.
Discussion
Fatigue was prevalent in this sample of PLWH, with 45% reporting clinically meaningful fatigue on at least one of the two fatigue items. Fatigue was also relatively constant for PLWH in our sample, not increasing or decreasing substantially over the 10 weeks between measurements of fatigue. These findings provide further evidence that fatigue is a significant problem among PLWH, and are consistent with previous data on the prevalence and severity of fatigue in this population (Barroso & Voss, 2013; Erlandson et al., 2014). Although we did not directly assess functional limitations in this study, prior research has shown that moderate to severe fatigue on the same two CES-D items does in fact predict frailty and activity restrictions (Fried et al., 2001) as well as greater risk for falls in middle-aged and older PLWH (Erlandson et al., 2012b).
Using data collected prospectively over a period of 10 weeks, initial fatigue predicted participants’ responses on five out of seven daily experience variables, consistent with the clinical observation that fatigue has a significant impact on the everyday lives of PLWH. This was particularly true for fatigue reflected in the experience of inertia, although two of the five daily experience variables predicted by that item were also significantly related to initial scores on the other CES-D fatigue item measuring effort.
The two daily experience variables not predicted by initial fatigue were HIV-related stigma and motivation for ART. These variables may have environmental or intrinsic causes that are less easily changed by PLWH’s experience of fatigue. In contrast, one can easily envision mechanisms by which fatigue might reduce perceived control in PLWH, worsen their mood, increase their perceived stress level, or decrease their coping efforts, all of which showed relationships to fatigue in the expected direction.
The effect of fatigue on social support was also in the expected direction, although there might be multiple mechanisms by which fatigue reduces perceived social support: for instance, PLWH who are fatigued might be less able to access existing social networks, might experience an actual reduction in available social support because they are less able to get out and engage in everyday life, or might have adequate support but perceive themselves not to as a result of their fatigue and associated poorer mood. Initial fatigue affected only the average level of each of these five daily experience variables and not their trajectory over time.
In addition to the predictive effects of fatigue on everyday experiences, the average scores of PLWH on two of the everyday experience scales also showed a reciprocal effect on subsequent fatigue. In this pattern of relationships, daily experience variables functioned both as phenomena (outcomes predicted by fatigue) and determinants (predictors of fatigue at a later point in time) according to the shape shifters model (Corwin et al., 2012). The second observation of fatigue occurred at a later point in time than the predictor variables, consistent with standard approaches to the study of causation. Within the shape shifters model, we were able to posit that such reciprocal relationships are common where biobehavioral phenomena such as disease symptoms are concerned. These relationships illustrate that fatigue is a complex biobehavioral phenomenon that responds to experiential variables such as stigma, social support, and life stressors, in addition to biological factors like HIV disease and side effects of ART.
Of particular interest in this study’s findings were the roles of stress and social support, both of which showed a shape shifter pattern of being predicted by fatigue and in turn predicting subsequent fatigue. The symptom and daily experience variables were mutually exacerbating, a classic self-perpetuating cycle pattern. Fatigue may make everyday stressors more salient and disturbing, or it may reduce perceptions by PLWH of the social support they have available. Alternately, fatigue might reduce people’s functioning in a way that creates additional life problems, such as boredom, aches and pains, or loss of valued social roles. Stress and social support in turn predicted greater fatigue, which suggests that if the cycle is not interrupted, people will continue to experience further consequences and greater fatigue over time. Stress and social support may therefore be particularly important areas in which clinicians could intervene to reduce subsequent fatigue and prevent future disability.
Just as positive social support can have a protective effect, negative social interactions can have a harmful one. In the current study, HIV-related stigma was not predicted by fatigue, but when it did occur, PLWH also reported greater subsequent fatigue. Stigma could be a source of stress, and might be ameliorated by social support, so it is conceivable that this variable might interact with the other momentary state variables that were found to predict fatigue. Unfortunately, we could not directly test such effects of combinations of variables, due to sample size limitations. Nevertheless, decreasing perceived stigma may be an additional way to reduce PLWH’s subsequent perceptions of fatigue.
Limitations and Directions for Future Research
In this secondary data analysis, fatigue data were collected at only two time points and were measured with individual items rather than on a multi-item instrument with well-established reliability and validity. This is a substantial limitation, particularly given that the two fatigue items were not highly inter-correlated and in fact showed different patterns of relationship to other variables in the study. Both of the CES-D items analyzed in this study have been used to measure fatigue in prior research with PLWH (Erlandson et al., 2012a,b, 2013; Kooij et al., 2016), and have been found to correlate with clinical outcomes related to fatigue (Fried et al., 2001). Nevertheless, future research using a more psychometrically sound fatigue measure would strengthen conclusions.
Additionally, longitudinal data from ecological momentary assessment do not provide conclusive evidence of causation. Two of Hume’s (1748/1993) criteria for causation are present—correlation and temporal order—but the third criterion of a clear mechanism for causation is still open to debate. Logical relationships between the predictor and criterion variables, including the fact that all effects were in the expected direction, tend to strengthen a causal argument. Nevertheless, definitive evidence of causation requires an experimental study.
Finally, the sample size in this study was moderate to small, especially after PLWH with too few daily survey data points for analysis or a lack of follow-up data were removed from the sample. Results therefore, may be sample-dependent, so additional research is recommended. PLWH were demographically diverse, and it is important to note that there were no significant differences between those who participated in the study and those who declined to participate, a finding that argues against selection bias despite the smaller than expected sample size. Furthermore, statistical power was sufficient to detect moderate effects even after attrition.
Implications for Research and Practice
Results of the current study confirm previous research showing that fatigue is a clinically important problem among PLWH. Clinicians should take PLWH fatigue symptoms seriously because these may predict disease progression (Cook, Schmiege, McClean, Aagaard, & Kahook, 2011) in addition to functional limitations (Erlandson et al., 2012a). Based on results of the current study, PLWH’s experiences of everyday stress, social support, and stigma may be key to understanding and addressing the problem.
Further research is needed to establish the precise mechanisms, whether cognitive, emotional, or physiological, by which fatigue and everyday experiences affect one another, but the role of everyday stress and social support as both outcomes of fatigue and predictors of subsequent fatigue are especially noteworthy findings. Intervention research using psychological counseling approaches to address PLWH stress or social support is promising. Stress-management interventions have been found efficacious among PLWH (Scott-Sheldon, Kalichman, Carey, & Fielder, 2008), but have not been widely adopted because their impact is primarily psychological, rather than directly affecting participants’ CD4 levels. Nevertheless, such established cognitive-behavioral interventions could potentially have important benefits for PLWH by disrupting the self-perpetuating cycle by which fatigue predicts greater stress and reduced social support, each of which in turn predicts later fatigue.
Acknowledgments
This research was supported by grant #1R21 NR012918-01 from the National Institute for Nursing Research, with additional infrastructure support from the Colorado Clinical and Translational Research Center, grant #1UL1 RR025870-01 and from the University of Colorado College of Nursing. In the past 12 months, Dr. Cook, Dr. Schmiege, Dr. Jankowski, and Dr. Meek have each received grant support from NIH, and Dr. Cook and Ms. Starr have received support from HRSA. Dr. Cook has also received grant support from SAMHSA and CDC, has served as a consultant for Takeda Inc. and the Optometric Glaucoma Society, and has received both consulting fees and grant support from Academic Impressions Inc.
Footnotes
Conflicts of interest: The authors report no conflicts of interest in this study.
Contributor Information
Paul F. Cook, College of Nursing, University of Colorado, Campus Box C288-04, Aurora, CO 80045.
Kimberly R. Hartson, College of Nursing, University of Colorado, Aurora, CO.
Sarah J. Schmiege, College of Nursing, University of Colorado, Aurora, CO.
Catherine Jankowski, College of Nursing, University of Colorado, Aurora, CO.
Whitney Starr, School of Medicine, University of Colorado, Aurora, CO.
Paula Meek, College of Nursing, University of Colorado, Aurora, CO.
References
- Al-Dakkak I, Patel S, McCann E, Gadkari A, Prajapati G, & Maiese EM (2013). The impact of specific HIV treatment-related adverse events on adherence to antiretroviral therapy. AIDS Care, 25, 400–414. doi: 10.1080/09540121.2012.712667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso J, & Voss JG (2013). Fatigue in HIV and AIDS. Journal of the Association of Nurses in AIDS Care, 24, S5–S14. doi: 10.1016/j.jana.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Berger BE, Ferrans CE, & Lashley FR (2001). Measuring stigma in people with HIV. Research in Nursing & Health, 24, 518–529. doi: 10.1002/nur.10011 [DOI] [PubMed] [Google Scholar]
- Berkman LF, Berkman CS, Kasl S, Freeman DH, Leo L, Ostfeld AM, … Brody JA (1986). Depressive symptoms in relation to physical health and functioning in the elderly. American Journal of Epidemiology, 124, 372–388. [DOI] [PubMed] [Google Scholar]
- Bhat VG, Ramburuth M, Singh M, Titi O, Antony AP, Chiya L, … Msengana M (2010). Factors associated with poor adherence to anti-retroviral therapy in patients attending a rural health centre in South Africa. European Journal of Clinical Microbiology & Infectious Diseases, 29, 947–953. doi: 10.1007/s10096-010-0949-4 [DOI] [PubMed] [Google Scholar]
- Chesney MA (2006). The elusive gold standard: Future perspectives for HIV adherence assessment and intervention. Journal of Acquired Immune Deficiency Syndromes, 43, S149–S155. doi: 10.1097/01.qai.0000243112.91293.26 [DOI] [PubMed] [Google Scholar]
- Cook PF, McElwain CJ, & Bradley-Springer L (2010). Feasibility of a daily electronic survey to study prevention behavior with HIV-infected individuals. Research in Nursing & Health, 33, 221–234. doi: 10.1002/nur.20381 [DOI] [PubMed] [Google Scholar]
- Cook PF, Schmiege SJ, McClean M, Aagaard L, & Kahook MY (2011). Practical and analytic issues in the electronic assessment of adherence. Western Journal of Nursing Research, 34, 598–620. doi: 10.1177/0193945911427153 [DOI] [PubMed] [Google Scholar]
- Cook PF, Schmiege SJ, Starr W, Carrington JM, & Bradley-Springer LA (2015). Prospective state and trait predictors of daily medication adherence behavior in HIV. Manuscript Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PF, Sousa KH, Matthews EE, Meek PM, & Kwong J (2011). Patterns of change in symptom clusters with HIV disease progression. Journal of Pain and Symptom Management, 42, 12–23. doi: 10.1016/j.jpainsymman.2010.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin EJ, Meek P, Cook P, Lowe N, & Sousa K (2012). Shape shifters: Biobehavioral determinants and phenomena in symptom research. Nursing Outlook, 60, 191–197. doi: 10.1016/j.outlook.2012.04.008 [DOI] [PubMed] [Google Scholar]
- Deeks SG, Tracy R, & Douek DC (2013). Systemic effects of inflammation on health during chronic HIV infection. Immunity, 39, 633–645. doi: 10.1016/j.immuni.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson KM, Allshouse AA, Jankowski CM, Duong S, MaWhinney S, Kohrt WM, & Campbell TB (2012a). Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clinical Trials, 11, 287–290. doi: 10.1310/hct1306-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson KM, Allshouse AA, Jankowski CM, Duong S, MaWhinney S, Kohrt WM, & Campbell TB (2012b). Risk factors for falls in HIV-infected persons. Journal of Acquired Immune Deficiency Syndromes, 61, 484–489. doi: 10.1097/QAI.0b013e3182716e38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, & Campbell TB (2013). Functional impairment is associated with low bone and muscle mass among persons aging with HIV-infection. Journal of Acquired Immune Deficiency Syndromes, 63, 209–215. doi: 10.1097/QAI.0b013e318289bb7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson KM, Schrack JA, Jankowski CM, Brown TT, & Campbell TB (2014). Functional impairment, disability, and frailty in adults aging with HIV-infection. Current HIV/AIDS Reports, 11, 279–290. doi: 10.1007/s11904-014-0215-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SB, Lu M, Glaze DG, Reuben JM, Harris LL, Cohen EN, … Shearer WT (2012). Associations of cytokines, sleep patterns, and cognitive function in youth with HIV infection. Clinical Immunology, 144, 23. doi: 10.1016/j.clim.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, … the Cardiovascular Health Study Collaborative Research Group. (2001). Frailty in older adults. Journal of Gerontology: Medical Sciences, 56A, M146–M156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- Gay C, Portillo CJ, Kelly R, Coggins T, Davis H, Aouizerat BF, … Lee KA (2011). Self-reported medication adherence and symptom experience in adults with HIV. Journal of the Association of Nurses in AIDS Care, 22, 257–268. doi: 10.1016/j.jana.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaker EL (2012). Why researchers should think “within-person”: A paradigmatic rationale. In Mehl MR & Conner TS (Eds.), Handbook of research methods for studying daily life. (pp. 43–61). New York: Guilford. [Google Scholar]
- Hertzog C, Van Alstine J, Usala PD, Hultsch DF, & Dixon R (1990). Measurement properties of the Center for Epidemiological Studies Depression scale (CES-D) in older populations. Psychological Assessment, 2, 64–72. doi: 10.1037/1040-3590.2.1.64 [DOI] [Google Scholar]
- Herzog TA, & Blagg CO (2007). Are most precontemplators contemplating smoking cessation? Health Psychology, 26, 222–231. doi: 10.1037/0278-6133.26.2.222 [DOI] [PubMed] [Google Scholar]
- Holm JE, & Holroyd KA (1992). The Daily Hassles Scale (Revised): Does it measure stress or symptoms? Behavioral Assessment, 14, 465–482. [Google Scholar]
- Hox J (2002). Multilevel analysis: Techniques and applications. Mahwah, NJ: Erlbaum. [Google Scholar]
- Hume D (1748/1993). An enquiry concerning human understanding (2nd ed.). Indianapolis, IN: Hackett. [Google Scholar]
- Jong E, Oudhoff LA, Epskamp C, Wagner MN, van Duijn M, Fischer S, & van Gorp E (2010). Predictors and treatment strategies for HIV-related fatigue in the combined antiretroviral therapy era. AIDS, 24, 1387–1405. doi: 10.1097/QAD.0b013e328339d004 [DOI] [PubMed] [Google Scholar]
- Kamarck TW (1998). The diary of ambulatory behavioral states. In Krantz DS & Baum A (Eds.), Technology and methods in behavioral medicine (pp. 163–185). Mahwah, NJ: Erlbaum. [Google Scholar]
- Kim HJ, Barsevick AM, Fang CY, & Miaskowski C (2012). Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nursing, 35, E1–E20. doi: 10.1097/NCC.0b013e318233a811 [DOI] [PubMed] [Google Scholar]
- Kooij KW, Wit FWNM, Schouten J, van der Valk M, Godfried MH, Stolte IG, … the AGEhIV Cohort Study Group. (2016). HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. AIDS, 30, 241–250. doi: 10.1097/QAD.0000000000000910 [DOI] [PubMed] [Google Scholar]
- Liu H, Golin CE, Miller JG, Hays RD, Beck CK, Sanandaji S, … Wenger NS (2001). A comparison study of multiple measures of adherence to HIV protease inhibitors. Annals of Internal Medicine, 134, 968–977. doi: 10.7326/0003-4819-134-10-200105150-00011 [DOI] [PubMed] [Google Scholar]
- Nichols JE, Speer DC, Watson BJ, Watson MR, Vergon TL, Vallee CM, & Meah JM (2002). Aging with HIV: Psychological, social, and health issues. New York: Academic Press. [Google Scholar]
- Pathai S, Bajillan H, Landay AL, & High KP (2014). Is HIV a model of accelerated or accentuated aging? Journal of Gerontology and Medical Science, 69, 833–842. doi: 10.1093/gerona/glt168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CED-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Schmiege SJ, Meek P, Bryan AD, & Petersen H (2012). Latent variable mixture modeling. Nursing Research, 61, 204–212. doi: 10.1097/NNR.0b013e3182539f4c [DOI] [PubMed] [Google Scholar]
- Scott-Sheldon LAJ, Kalichman SC, Carey MP, & Fielder RL (2008). Stress management interventions for HIV+ adults: A meta-analysis of randomized controlled trials, 1989 to 2006. Health Psychology, 27, 129–139. doi: 10.1037/0278-6133.27.2.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa KH, Kwok OM, & Tann SS (2004). Testing of a measurement model for HIV-AIDS symptom status. Journal of the Association of Nurses in AIDS Care, 17, 36–46. doi: 10.1016/j.jana.2006.01.004 [DOI] [PubMed] [Google Scholar]
- Stone AA, & Neale JM (1984). New measure of daily coping. Journal of Personality and Social Psychology, 46, 892–906. doi: 10.1037/0022-3514.46.4.892 [DOI] [PubMed] [Google Scholar]
- Swan M (2013). The quantified self: Fundamental disruption in big data science and biological discovery. Big Data, 1, 85–99. doi: 10.1089/big.2012.0002 [DOI] [PubMed] [Google Scholar]
- Walls TA, Jung H, & Schwartz JE (2006). Multilevel models for intensive longitudinal data. In Walls TA & Schafer JL (Eds.), Models for intensive longitudinal data. (pp. 3–37). New York: Oxford University Press. [Google Scholar]
- Walls TA, & Schafer JL (2006). Introduction: Intensive longitudinal data. In Walls TA & Schafer JL (Eds.), Models for intensive longitudinal data (pp. xi–xxii). New York: Oxford University Press. [Google Scholar]


