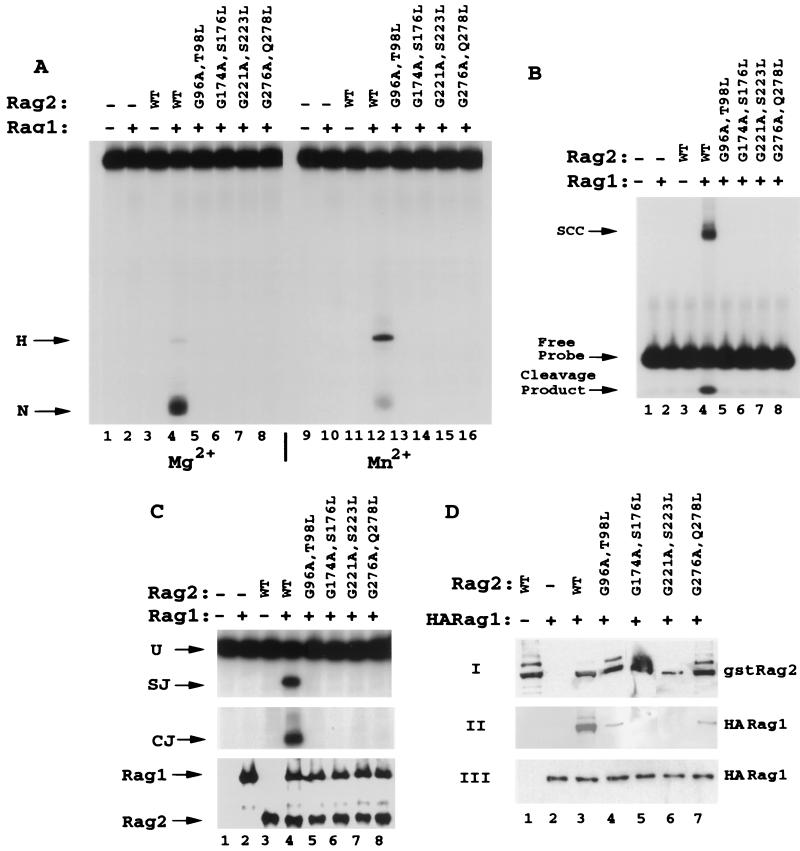
FIG. 4.
Mutations in RAG2 glycine-serine-threonine-rich regions affect the interaction with RAG1 and concomitantly block RSS binding and nicking. (A) Double mutations in repeats 2 (G96A T98L), 3 (G174A S176L), 4 (G221A S223L), and 5 (G276A Q278L) were generated by substituting the second glycine of the glycine doublets as well as a serine or threonine residue 2 amino acids C-terminal to the glycine (except in the case of the fifth repeat, which has glutamine at this position [Fig. 1]). GST-RAG2 fusions (amino acids 1 to 383) were purified and tested for 12 RSS nicking and hairpin activity. In both Mg2+ (lanes 1 to 8) and Mn2+ (lanes 9 to 16), all four mutant proteins were entirely inactive for either nicking or hairpinning the 12 RSS compared to the wild type (WT). (B) Mutant RAG2 proteins were assayed for the capacity to form SCC along with wild-type GST-RAG1 on the 12 RSS. As with 12 RSS cleavage, all mutants were inactive for SCC formation (lanes 5 to 8). Cleavage products can be visualized below the free probe. (C) Mutants were tested for recombination activity on substrate pJH200 by PCR analysis for signal joints (SJ) and coding joints (CJ). None of the four mutants (lanes 5 to 8) generated detectable recombination products (SJ or CJ). Aliquots of the cell lysates were evaluated for protein expression by Western blot analysis. (D) Interaction between GST-RAG2 and HA-tagged RAG1 was monitored as described in the legend for Fig. 3C.