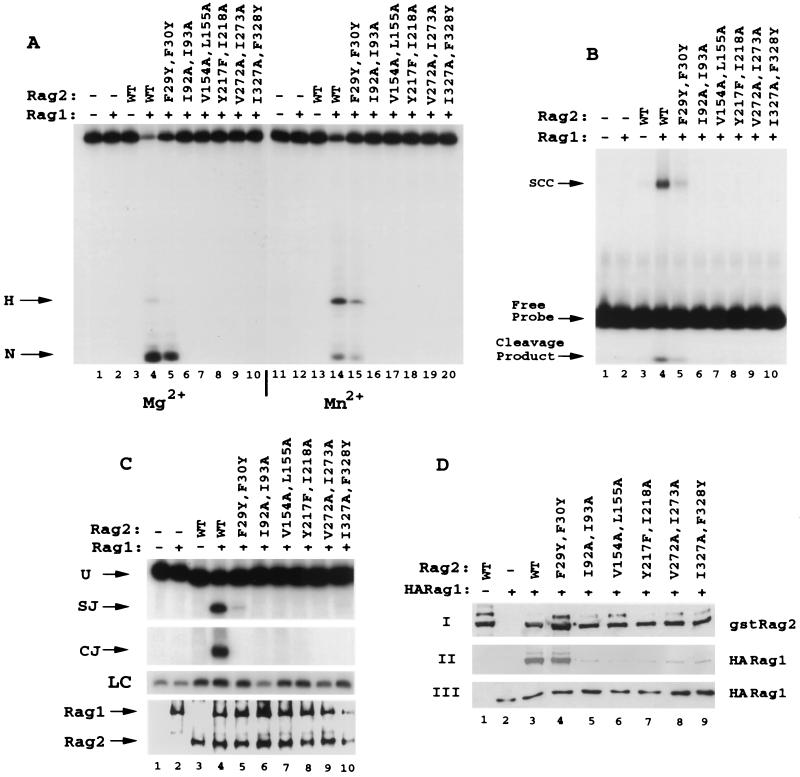
FIG. 5.
Mutation of most of the hydrophobic residues in the predicted second β-strand of repeats 1 to 6 of RAG2 decreases the binding of RAG1, with parallel effects on in vitro RSS binding and cleavage. (A and B) Conservative double mutations (F29Y F30Y, I92A I93A, V154A L155A, Y217F I218A, V272A I273A, and I327A F328Y) (Fig. 1) in the hydrophobic regions of kelch repeats 1 through 6 were expressed and purified as GST fusion proteins and tested in vitro for 12 RSS cleavage in Mg2+ (lanes 1 to 10) and Mn2+ (lanes 11 to 20) (A) or for SCC formation with the 12 RSS (B). (C and D) In vivo analysis of signal joint and coding-joint formation was conducted (C) in addition to analysis of complex formation between RAG1 and RAG2 (D). In panel C, loading controls (LC) performed in the linear range of the PCR are shown. WT, wild type.