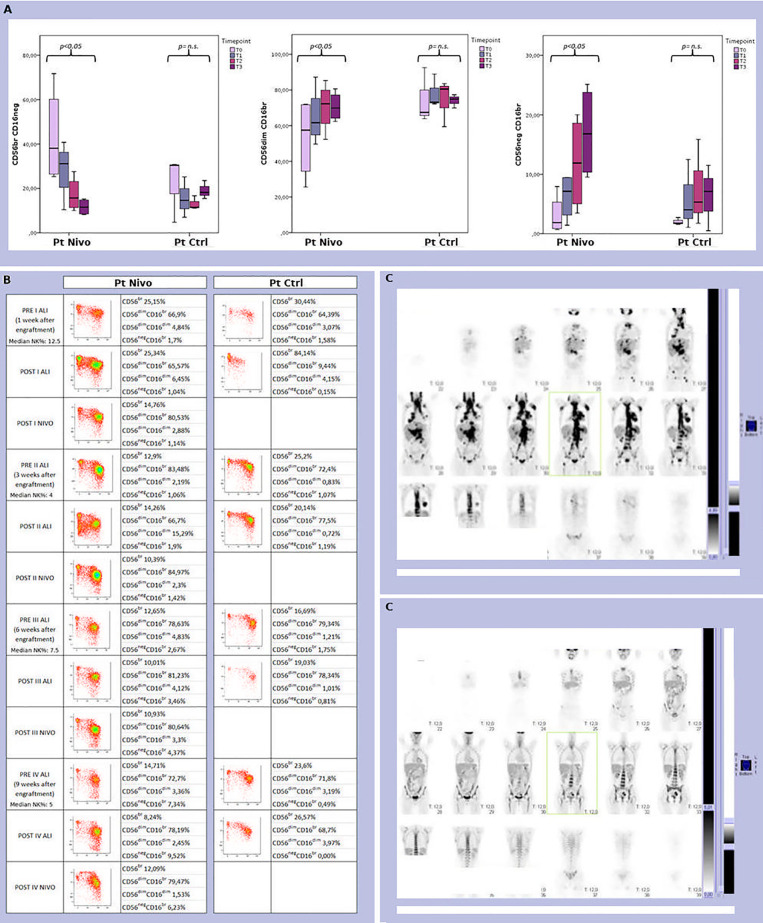
Figure 4.
(A) Analysis of the size and distribution of CD56brightCD16neg/dim, CD56dimCD16bright, and CD56negCD16bright NK cell subsets derived from peripheral blood of 4 treated (Pt Nivo) and 3 control (Pt Ctrl) patients at different time points after ASCT (t0: pre-treatment; t1: first post ALI + nivolumab for treated patients and first ALI for control patient; t2: second post ALI + nivolumab for treated patients and second ALI for control patient; t3: third post ALI + nivolumab for treated patients and third ALI for control patients). Data represent the mean ± 95% CI. p < 0.05 (B) Comparison of peripheral blood NK cell subsets distribution in two patients representative of the two cohort -patient receiving ALI + nivolumab (Pt Nivo); patient receiving ALI alone (control) (Pt Ctrl)- at the different steps indicated in the Figure. ns, not significant. (C) PET scan from the patient receiving ALI + nivolumab at enrollment (upper panel C) and at end of treatment (EOT) (lower panel C). All the patients shown in Figure 4 were seropositive for HCMV, but none of them experienced HCMV reactivation during all study period.