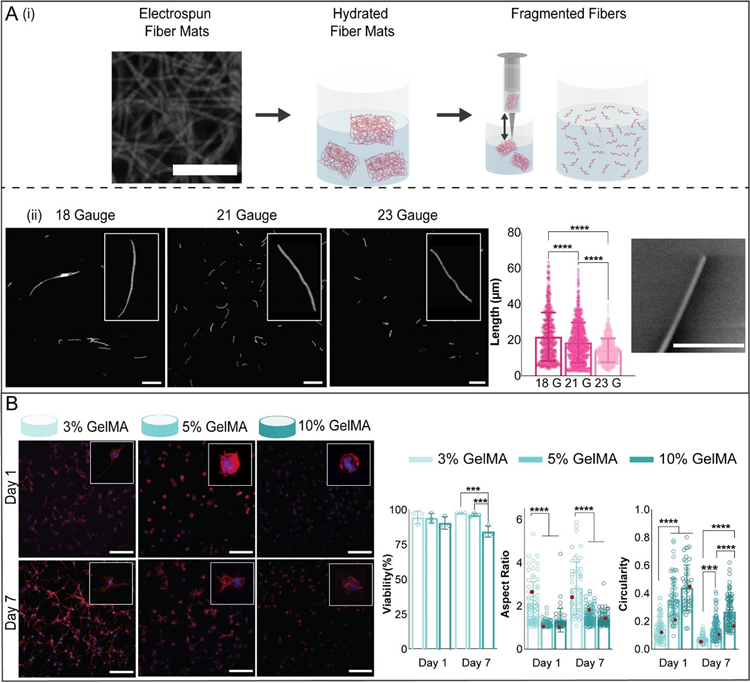
Figure 2.
Microfiber and hydrogel formulations. (A) (i) To fabricate microfibers, electrospun norbornene-HA (NorHA) fiber mats (scale bar 10 μm) were crosslinked, hydrated, sectioned into pieces, and fragmented into microfibers by repeatedly passing through a needle. (ii) Fiber length was varied by varying the needle gauge used to shear fibers. Representative images of suspensions of fragmented fibers for three different needle gauges are shown (left, scale bar 0.1 mm, along with quantification of fiber length (middle) and an SEM image showing the end of a fragmented fiber (right, scale bar 2 μm, mean ± s.d., one-way ANOVA with Tukey post hoc, ****p ≤ 0.0001). (B) Representative images of meniscal fibrochondrocytes (MFCs) stained for F-actin (red) and cell nuclei (blue) (200 μm z-stacks, scale bars 0.1 mm) after culture in 3, 5, and 10% GelMA hydrogels for 1 and 7 days. Quantification of MFC viability (analyzed with live-dead staining), aspect ratio, and circularity after 1 and 7 days of culture (3% GelMA: n= 52 (Day 1), 68 (Day 7) cells; 5% GelMA: n= 64 (Day 1), 86 (Day 7) cells; 10% GelMA: n= 46 (Day 1), 89 (Day 7) cells; 3 biologically independent experiments, mean ± s.d., two-way ANOVA with Bonferroni post hoc ****p ≤ 0.0001, ***p ≤ 0.001, red dots indicate measurements for magnified images).