FIGURE 2.
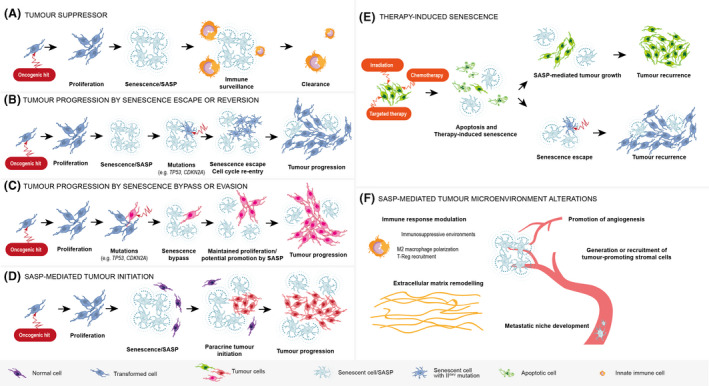
Proposed roles of senescence and SASP in tumourigenesis. (A) Tumour suppressor mechanism. Oncogenic signalling leads to transient proliferation followed by senescence induction (oncogene‐induced senescence). Senescent cells activate SASP and attract immune cells that clear them from the tissues, thus preventing subsequent tumour development. (B) Tumour progression by senescence escape or reversion. Following senescence induction, one cell accumulates further mutations (e.g. TP53, encoding p53, or CDKN2A, encoding p16INK4a) resulting in senescence escape, cell‐cycle re‐entry, proliferation and tumour formation. (C) Tumour progression by senescence bypass or evasion. Upon oncogenic signalling and initial proliferation burst, most of the cells become senescent but one cell continues proliferating due to pre‐existing mutations in key senescence regulators (e.g. p53 or p16) that prevents the establishment of a senescence response. (D) SASP‐mediated tumour initiation. Senescent cells, through the SASP, create a pro‐tumourigenic microenvironment that leads to cell transformation of a non‐tumour cell and tumour formation. (E) Therapy‐induced senescence. Following irradiation, chemotherapy and targeted therapy, most of the cells in the tumour bed are killed (e.g. by apoptosis) or induced into senescence with some cells being unaffected (green cell). Senescent cells will eventually contribute to tumour recurrence either in a paracrine manner through SASP‐mediated tumour growth, or by senescence escape or bypass. (F) SASP‐mediated tumour microenvironment alterations. Senescent cells, through the SASP, can remodel the tumour microenvironment. For instance, by (i) modulating the immune response to create immunosuppressive environment (e.g. M2 macrophage polarisation, T‐reg recruitment), (ii) driving extracellular matrix remodelling and tumour vascularisation and (iii) promoting the development of metastatic niches
