FIGURE 4.
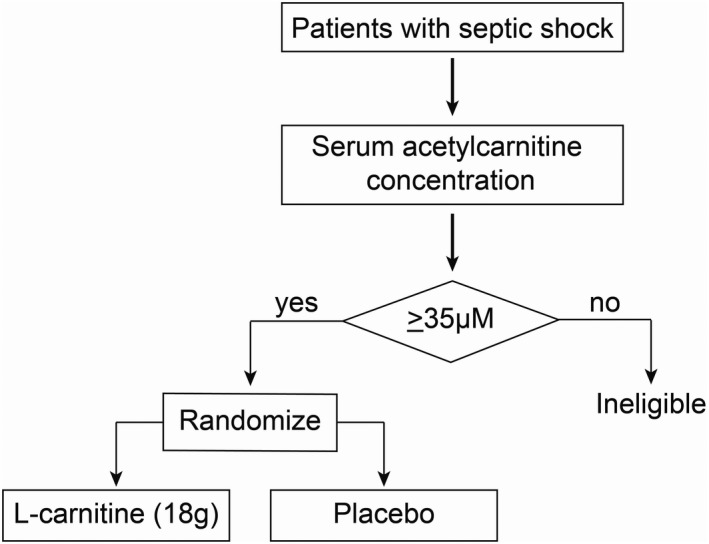
A clinical trial enrichment strategy could optimize clinical trial design for heterogeneous critical illnesses like sepsis. An example of a scheme for a hypothetical phase III clinical trial of supplement L‐carnitine for the treatment of septic shock that uses an a priori determined acetylcarnitine (C2) threshold concentration to determine whether a patient is enrolled and randomized to receive either L‐carnitine (18 g) or placebo
