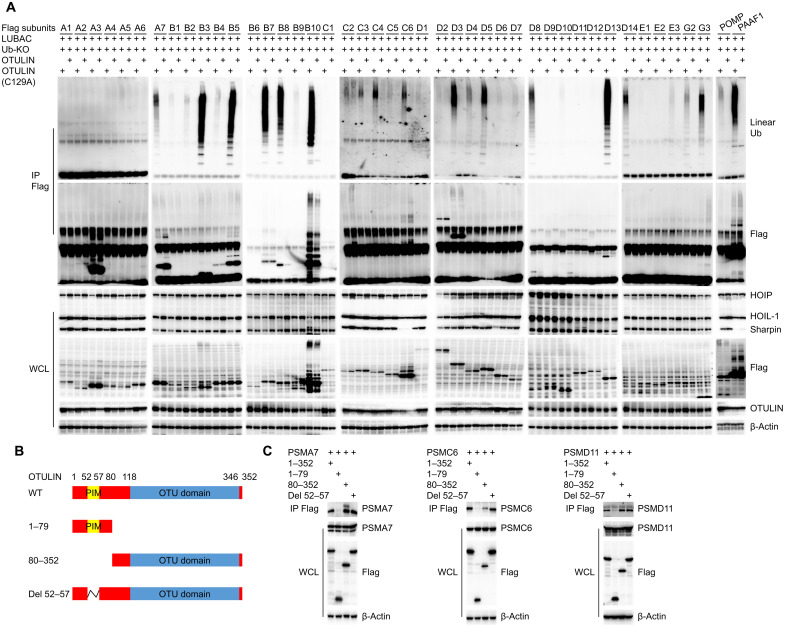
Fig. 3. Proteasome subunits as substrates of OTULIN deubiquitinase activity.
(A) Identification of proteasome subunits that are regulated by OTULIN-mediated deubiquitination of linear ubiquitin using immunoprecipitation (IP). LUBAC complex consists of the catalytic subunit HOIP and two accessory proteins: HOIL-1 and Sharpin. WCL, whole-cell lysate. Ub-KO, ubiquitin mutant with all lysines mutated to arginines, which only forms linear polyubiquitin chains. (B) Schematic illustration of the different OTULIN deletion constructs that are used in experiments shown in (C). (C) Immunoprecipitation of 293T cells transfected with the indicated OTULIN constructs and various proteasome subunits provides evidence that the interaction between OTULIN and proteasome subunits relies on the OTU domain.