Abstract
Ferritin is a known inflammatory biomarker in COVID-19. However, many factors and co-morbidities can confound the level of serum ferritin. This current metaanalysis evaluates serum ferritin level in different severity levels in COVID-19. Studies evaluating serum ferritin level in different clinical contexts (COVID-19 vs. control, mild to moderate vs. severe to critical, non-survivor vs. survivor, organ involvement, ICU and mechanical ventilation requirement) were included (total 9 literature databases searched). Metaanalysis and metaregression was carried out using metaphor “R” package. Compared to control (COVID-19 negative), higher ferritin levels were found among the COVID-19 patients [SMD −0.889 (95% C.I. −1.201, −0.577), I2 = 85%]. Severe to critical COVID-19 patients showed higher ferritin levels compared to mild to moderate COVID-19 patients [SMD 0.882 (0.738, 1.026), I2 = 85%]. In meta-regression, high heterogeneity was observed could be attributed to difference in “mean age”, and “percentage of population with concomitant co-morbidities”. Non-survivors had higher serum ferritin level compared to survivors [SMD 0.992 (0.672, 1.172), I2 = 92.33%]. In meta-regression, high heterogeneity observed could be attributed to difference in “mean age” and “percentage of male sex”. Patients requiring ICU [SMD 0.674 (0.515 to 0.833), I2 = 80%] and mechanical ventilation [SMD 0.430 (0.258, 0.602), I2 = 32%] had higher serum ferritin levels compared to those who didn't. To conclude, serum ferritin level may serve as an important biomarker which can aid in COVID-19 management. However, presence of other co-morbid conditions/confounders warrants cautious interpretation.
Keywords: COVID-19, SARS-CoV2, Ferritin, Hyperferritin, Diagnosis, Prognosis, Biomarker
Abbreviation: SARS-CoV2, Severe acute respiratory distress syndrome corona virus 2; COVID-19, Corona virus disease 2019; SMD, Standardized mean difference; MD, Mean difference
1. Introduction
Human ferritin is composed of two subunits, namely ferritin heavy chain (FTH) and ferritin light chain (FTL) [1]. The FTH chain has ferroxidase activity and oxidizes Fe2+ to Fe3+. Fe3+ then moves towards the nucleation site on the FTL chain and thus by acting in a synchronizing way, iron oxidation and core formation is carried out [2]. The ferritin units assemble in the form of a sphere with a cage inside it, the outer and inner diameter of which is 12 and 8 nm respectively. This nano-cage (stable at pH range 3 to 9) separates iron in the core from the outside environment and thus protects the body from harmful effects of excess free iron [2]. One ferritin molecule can store upto 5000 iron atoms [2].
The synthesis of ferritin is regulated by various “oxidant and antioxidant stimuli” e.g. nitrous oxide, glutathione and other “reactive oxygen species”. The ratio of expression of both FTH as well as FTL is influenced by inflammatory process [1]. Iron regulatory proteins 1 & 2 (IRP1 or IRP2) alter the translation of FTH and FTL mRNA by interacting through a regulatory region on the mRNA which is known as “iron response element” (IRE) in the “5′ untranslated region” (UTR) [1,3].
Serum ferritin, being an “acute phase reactant”, mirrors the degree of both chronic as well as acute inflammatory reaction inside the body. However there is uncertainty whether hyperferritinaemia is a result or mediator of inflammation [4]. A higher ferritin level indicates an activated monocyte-macrophage system. In monocytes and macrophages, synthesis of ferritin is responsive to alteration in cytokine status at both the transcriptional and translational level [1]. FTH modulate responses of macrophage to immune stimuli (e.g. cytokine profile) and promotes polarization towards M1 or M2 status which further governs the inflammatory status of the body [2]. Direct interaction between ferritin and lymphocyte function is also reported [1].
Hyperferritinaemia is observed across a range of inflammation driven disorders and it serves as a validated biomarker across different disease domains e.g. rheumatologic disorders, different cancers and inflammatory conditions [1]. However, how intracellular ferritin reaches serum is a matter of concern. Edeas M et al., 2010 postulated that hyper-ferritinemia may be due to leakage from damaged intracellular stores [4]. Once released from tissue stores, ferritin loses the inner iron content and gives rise to excess free iron [5]. Excess iron or iron repletion favours growth of many viruses [6]. Increased iron favour replication of hepatitis C virus (HCV) and iron loading in the settings of HCV is associated with poorer outcome. Similar results also reported in case of human immunodeficiency virus (H.I.V.) [6]. Many of the important SARS-CoV-2 regulatory and functional proteins use iron [7]. Excess iron can also induce fibrin polymerization [8,9] and induce a pro-coagulant state [5]. Previous literature reported occurrence of coagulopathy among severe COVID-19 patients [10,11]. While one hypothesis states that infection with SARS-CoV-2 results into direct interaction with haemoglobin (thus facilitates removal of iron), however this was later refuted by subsequent authors [12]. Again, the “role of iron chelation” in the context of COVID-19 is yet controversial [7,13].
COVID-19 represents a systemic inflammatory condition with elevation of pro-inflammatory markers (e.g. CRP, ESR) and pro-inflammatory cytokines e.g. IL-6, TNF-alpha etc. especially among patients with severe disease [[14], [15], [16]]. “Release of proinflammatory cytokines” (e.g. IL-6, IL-1B and TNF-alpha) [17], cellular damage, metabolic acidosis, associated ROS generation and secondary tissue damage are hypothesized mechanisms of high ferritin level in association with COVID-19 [17]. Individual studies reported that in patients with COVID-19, serum ferritin correlates with disease severity and its surrogates (CRP) [17].
Till now only single metaanalysis [18] evaluated the association between serum ferritin and COVID-19 severity and outcome (data collected upto 16th Aug, 2020), however heterogeneity among the studies were very high. In this regard, we conducted this metaanalysis (data included upto 30th April 2021) to evaluate the association between serum ferritin level and severity of disease, organ involvement, need of invasive ventilation and survival. In addition, in case of high heterogeneity, we have used metaregression and subgroup analysis to identify confounders and evaluate the causes of high heterogeneity and thus indicating a more rigorous methodology.
2. Material and method
2.1. Methods
This systematic review and metaanalysis was done according to “the fundamentals laid” in the “Cochrane Handbook for Systematic Reviews of Interventions” [19] and stated by “Preferred Reporting Items for Systematic reviews and Meta-Analysis” [20]. (PROSPERO registration number of the study is CRD42020212871).
2.2. Purpose
To evaluate the association between serum ferritin and severity and outcome of COVID-19.
2.3. Objectives
-
1.
Comparative evaluation of serum ferritin level between COVID-19 patients and control.
-
2.
Association between serum ferritin level and severity of COVID-19
-
3.
Association between serum ferritin level and survival in COVID-19.
-
4.
Association between serum ferritin level and requirement of mechanical ventilation (MV).
-
5.
Association between serum ferritin level and requirement of ICU.
-
6.
Association between serum ferritin level and different organ involvement in COVID-19 (heart, kidney, liver).
-
7.
Association between serum ferritin level and occurrence of thrombotic complications in COVID-19.
2.4. Inclusion criteria
-
1.
Study design: Observational studies (prospective, retrospective or ambispective) reporting serum ferritin level among patients with COVID-19
-
2.
Quality of study: Only good and fair quality studies were included (on the basis of risk of bias analysis).
-
3.
Age: Adult age group
-
4.
Sex: Both female and male sex.
2.5. Exclusion criteria
-
1.
Case report, case series
-
2.
Poor-quality studies (as decided by risk of bias analysis).
2.6. Case definition of COVID-19
The RT-PCR positive COVID-19 population is well defined. However, owing to low sensitivity of the diagnostic tests including RTPCR, false negativity [21] is an important problem and “clinically diagnosed RT-PCR negative COVID-19 is now increasingly being recognized [22,23]. In our study we used both RT-PCR positive COVID-19 and clinically diagnosed RT-PCR negative COVID-19 cases. COVID-19 Severity was defined as per predefined criteria laid down by WHO [24,25].
2.7. Search strategy
A comprehensive search of various databases (PubMed, Scopus, Embase, CINAHL, Web of Science, Science Direct, Google Scholar, Cochrane library and OVID) was performed from inception (1st November 2019) to 30th April 2021 without any language restriction. Apart from these, the references of the included studies were also screened for the possible inclusion. The search was conducted using the keywords: “corona virus disease-19”, “corona virus disease 2019”, “COVID-19”, “2019-nCoV”, “2019 nCoV”, “SARS-CoV2”, “ferritin” and “hyperferritin”.
2.8. Screening of studies
After search of databases, the articles were screened as per predefined inclusion/exclusion criteria for inclusion using title and abstract by KK & HK following which full text of the relevant articles were further screened. In case of discrepancy between two reviewers, the issue was resolved after consultation with BM and PS.
2.9. Data extraction
Two authors namely PS and HK independently extracted the data using “pre-tested data extraction form”.
2.10. Risk of bias (ROB) evaluation
The methodological quality of clinical studies was performed using the “New-castle Ottawa Scale” (NOS) [26]. Risk of bias was evaluated in three domains: selection, comparability and outcome [26]. The studies were converted to AHRQ standards (good, Fair and poor) on the basis of number of stars in each domains (across selection, comparability and outcome domain) as per existing standard methodology [26]. Good quality study is indicated by 3* or more selection and 1* or more in comparability and 2* or more in outcome domain. Fair quality study is indicated by 2* in selection and 1* or more in comparability and 2* or more in outcome domain. Poor quality: 0/1 * in selection OR 0 * in comparability or 0/1 8 in outcome domain [26]. Only good and fair quality studies were included in the metaanalysis. (Supplementary Table 1).
2.11. Statistical analysis
While pooling the results from different studies, in case of continuous outcomes presented in same scale, we used mean difference (MD) to get the point estimate. Standardized mean difference (SMD) was used for combining continuous data presented in different scales. Dichotomous outcomes were reported as risk ratios (RRs). Meta-analysis of dichotomous data was performed using “Mantel Haenszel method” and continuous data was performed using “inverse variance method”. 95% confidence interval was calculated for all the point estimates. Heterogeneity among the study results were evaluated by I2 statistics [19]. In case of low to moderate heterogeneity (<50%), “fixed-effect model” was used for pooling of data whereas in presence of significant heterogeneity (>50%) “random-effects model” was used [19]. In case of substantial and significant heterogeneity, the cause of high heterogeneity was investigated using subgroup analysis and meta-regression as described below. We used metaphor R package [27], RevMan [28] and SPSS (IBM, Newyork) for the analysis of data [29].
2.12. Subgroup analysis
We conducted subgroup analysis and metaregression to evaluate the etiology of heterogeneity. Subgroup analysis was carried on the basis of study design (prospective, retrospective, ambispective, unclassifiable), COVID-19 population versus COVID-19 in special population (kidney transplant, patients with specific disease e.g. rheumatoid arthritis, neurological disease etc.) and data used for analysis (original data versus derived data). Many studies have reported serum ferritin level among different comparisons as mean ± SD (termed original data in our study). However, many studies reported median (IQR). In case of median (IQR), mean ± SD was derived (termed as derived data) for use in the metaanalysis using method as described by Wan et al, 2014 [30].
2.13. Meta-regression
We used meta-regression to evaluate the potential source of heterogeneity among the included studies. As many factors (e.g. age, sex, smoking and co-morbid diseases) affect the level of serum ferritin [31], univariate meta-regression analysis was done to observe the effect of these confounders on the final result. P < 0.05 indicates a significant association and slope of the balloon plot regression line indicates the direction of association [29]. Metaregression was carried out in case when there were 10 or more studies against a variable [19].
2.14. Publication bias
“Publication bias” was evaluated using funnel plot and by using Egger's regression test [19].
3. Result
3.1. Details of included studies
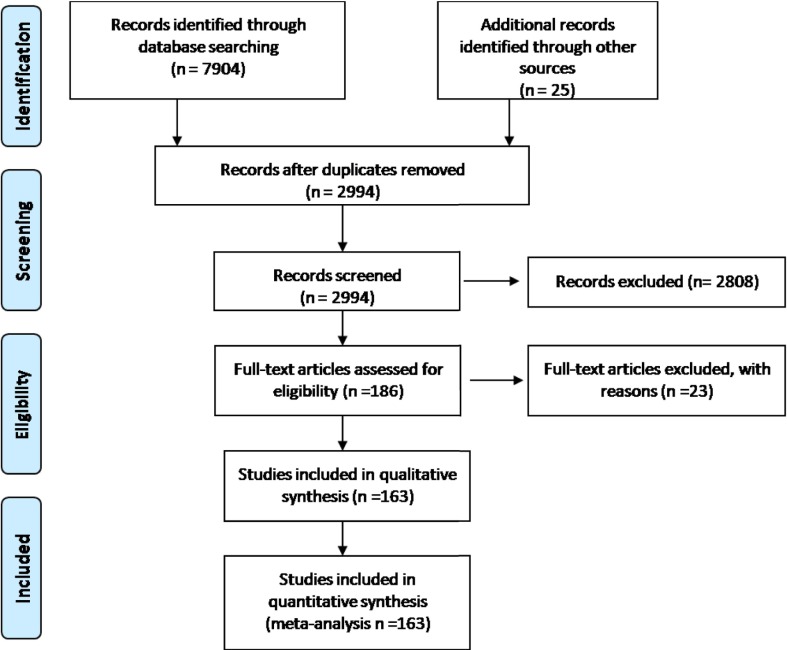
A total of 7929 studies were obtained after searching nine data bases. After removal of duplicates, 2994 studies were obtained, which were further screened using title and abstract. Among all, full text screening was done for 186 relevant articles. Finally a total of 163 studies fulfilling “predefined inclusion/exclusion criteria” were included in final analysis. [Detail of included studies shown in Supplementary table 1 and PRISMA flow chart is showed in Fig. 1 ].
Fig. 1.
PRISMA flow chart of the included studies.
3.2. Risk of bias among the included studies
Details of risk of bias evaluation among the included studies are shown in Supplementary table 1.
3.3. Serum ferritin: COVID-19 negative VS. COVID-19 positive
A total of 6 studies have reported “COVID-19 vs. non-COVID-19 patient” data [[32], [33], [34], [35], [36]]. Serum ferritin level was significantly higher among COVID-19 positive patients [6 studies, standardized mean difference (SMD) −0.889 (95% C.I. −1.201 to −0.577)]. Although heterogeneity among the included studies was high (I2 = 85%), however, the directions of effect of all the included studies are the same. [Data shown in Suppl. Fig. 1].
3.3.1. COVID-19 vs. control: subgroup analysis and metaregression analysis
To investigate the possible cause of high heterogeneity, subgroup analysis was conducted as pre-specified. However, sub-group analysis couldn't give reasonable explanation to the high heterogeneity observed. As number of studies was limited, we did not conduct a meta-regression analysis to investigate the cause of heterogeneity.
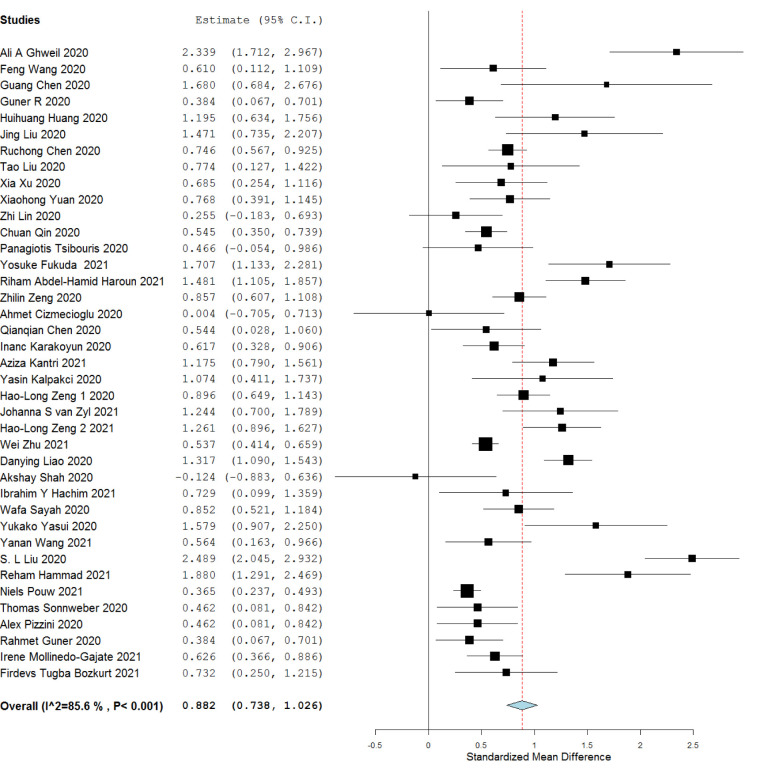
3.4. Severe and critical (SC) VS. mild to moderate disease (MM):
Serum ferritin level was significantly higher in the severe to critical patients compared to mild to moderate category patients [39 studies, SMD 0.882 (0.738 to 1.026) I2 = 85%]. Heterogeneity among the included studies was high (I2 = 85%). Data showed in Fig. 2 .
Fig. 2.
Serum Ferritin level among severe and critical versus mild and moderate patients.
3.4.1. Investigation of heterogeneity: subgroup and metaregression analysis
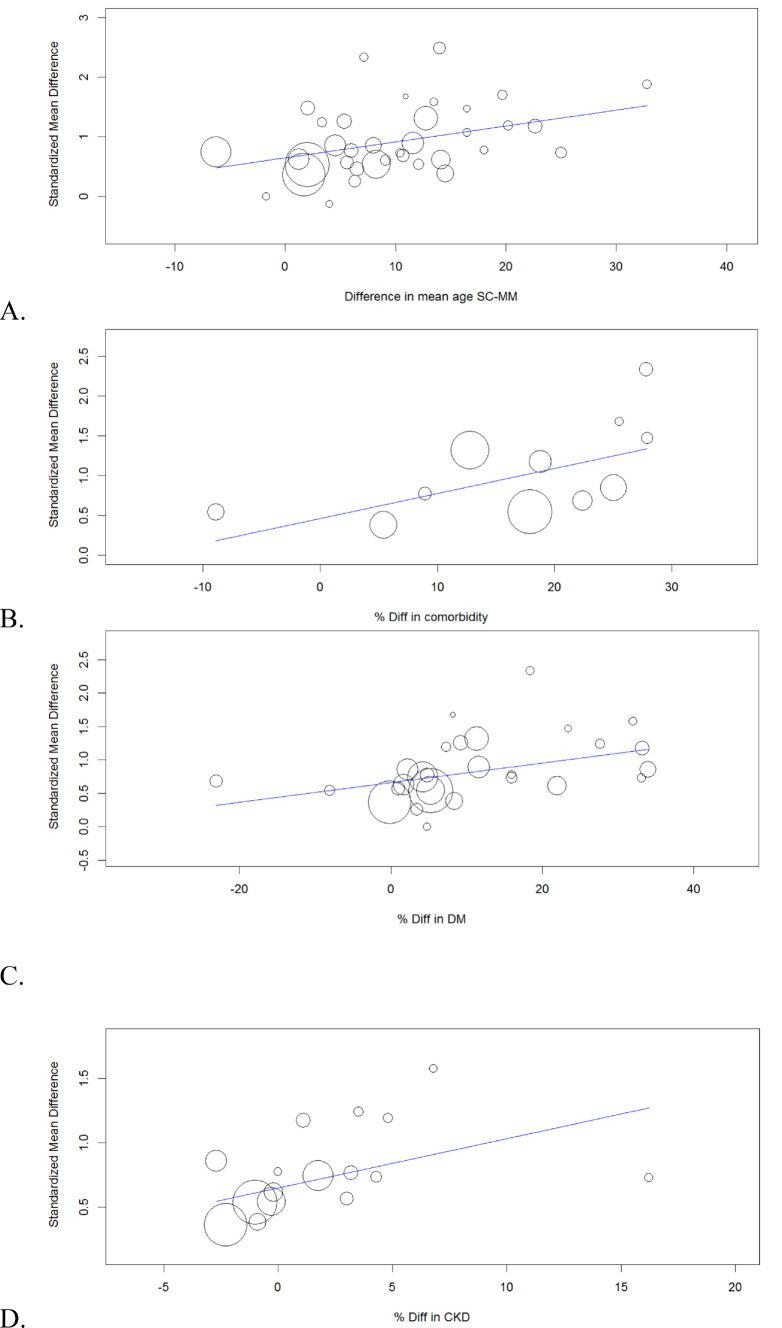
To investigate the possible cause of high heterogeneity, subgroup analysis was conducted as pre-specified. However, subgroup analysis couldn't give a definite conclusion on the etiology of heterogeneity. We conducted a metargression analysis to evaluate the possible cause of this heterogeneity. The detail of meta-regression analysis is shown in Suppl. Table 2. Difference (diff.) in mean age and diff. in percentage of patients with any co-morbidity between the two groups (SC vs. MM) was found to be significantly associated with the mean difference in ferritin level. The balloon plots of significant co-variates are showed in Fig. 3 .
Fig. 3.
Severe and critical versus mild and moderate: Meta-regression analysis to evaluate the impact of imbalance between the two groups in terms of mean age (A), Difference in percentage of population with co-morbidity (B), difference in percentage of population with diabetes mellitus (C) and difference in percentage of population with chronic kidney disease (D) and its impact on mean difference in serum ferritin level.
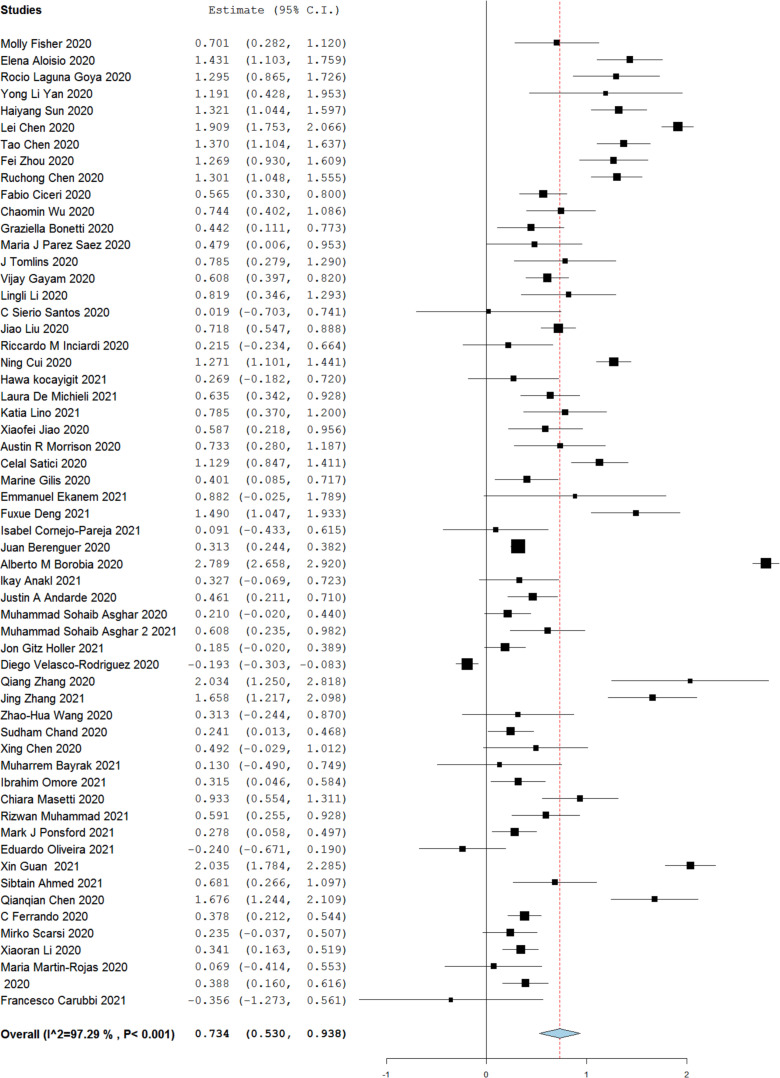
3.5. Non-survivor (NS) versus survivor (S)
A total of 57 studies reported serum ferritin levels among “survivors vs. non-survivors”. A higher serum ferritin level was noted in non-survivors (NS) as compared to survivors [SMD 0.734 (0.530–0.938)]. However, high heterogeneity was seen among the included studies (I2 = 97%). (Fig. 4 )
Fig. 4.
Serum ferritin level among non-survivor versus survivor.
3.5.1. Non survivor vs. survivor: exploring heterogeneity: Subgroup and metaregression analysis
To investigate the possible cause of high heterogeneity, subgroup analysis was conducted as pre-specified. However, subgroup analysis couldn't give a definite conclusion on the etiology of heterogeneity. In metaregression analysis, mean difference in age and % difference of male sex correlated significantly with the mean difference in ferritin level between the two groups. Data showed in Supplementary Table 3 and Suppl. Figs. 2 & 3.
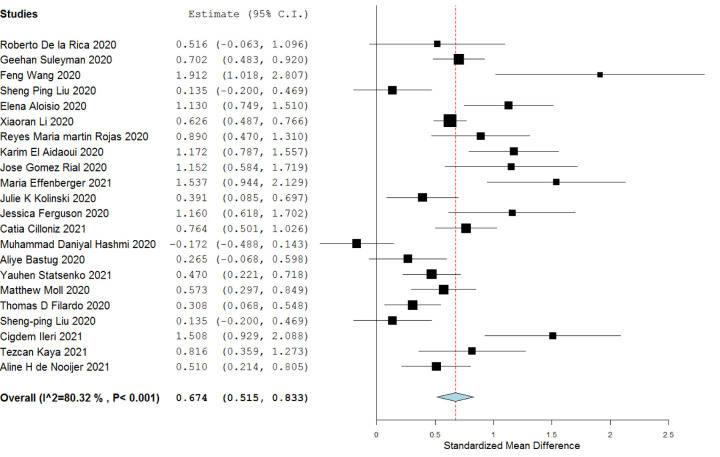
3.6. Requirement of ICU
Patients requiring ICU had a higher serum ferritin level than patients who did not require the same [SMD 0.674 (0.515 to 0.833)]. Heterogeneity among the included studies were high (I2 = 80%), however, the direction of effect was same in all the studies except the study by Hashemi MD et al. 2020. [Data showen in Fig. 5 ]
Fig. 5.
Serum ferritin level among patients who required ICU versus those who didn't.
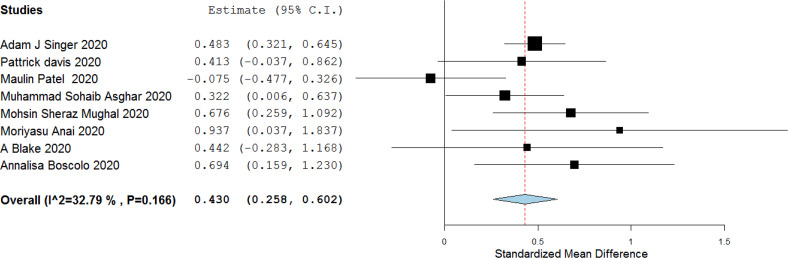
3.7. Requirement of mechanical ventilation (MV)
A total of 8 studies reported ferritin level among patients requiring and not requiring mechanical ventilation. Standardized mean difference between the two groups was 0.430 (0.258 to 0.602), I2 = 32%] indicating association of higher level of serum ferritin and requirement of mechanical ventilation. [Data showen in Fig. 6 ].
Fig. 6.
Serum ferritin level among patients who required MV versus who didn't require MV.
3.8. Serum ferritin level and organ involvement (hepatic/myocardial/renal) in Covid-19
3.8.1. Organ involvement: liver
Liver enzyme elevation is common in COVID-19. Phipps et al. [37] reported that, both initial and peak serum ferritin levels were higher in patients with ALT 2–5 time of normal and ALT >5 times of upper limit of normal compared to those with ALT<2XULN (p < 0.001). In the study by Ramachandran et al. [38], although the level of ferritin was higher in the ALT elevation group, however the difference was not statistically significant. Similar findings of higher ferritin levels among COVID-19 patients with liver injury is also reported by Da et al., 2021 [39], Mishra et al., 2020 [40]. However, in a small sample sized study no difference was observed by Zhang et al., 2020 [41]. As the heterogeneity among the study results were high, the results were not pooled.
3.8.2. Organ involvement: heart
No data regarding COVID-19 related cardiac involvement vs. those without cardiac involvement.
3.8.3. Organ involvement: kidney
Mohammad et al. [42] (single study) reported that, serum ferritin levels were higher in patients with COVID-19 related acute kidney injury [(AKI) median 1016 ng/mL (IQR 516–2534)] compared to those without AKI [median 680 (IQR 315 to 1416)]. Similar findings are also reported by Azam et al. [43], Lee et al. [44], Hansrivijit et al. [45] and Peng et al. [46].
3.9. Serum ferritin and occurrence of thrombotic complications: (thrombotic complications: absent vs. present)
When the ferritin level was compared without and with thrombotic complications, significantly higher ferritin level was noted in patients with thrombotic complications [Mean difference −614.75 (−773.171 to −456.329), I2 = 0%]. (Suppl. Fig. 4)
3.10. Publication bias
Publication bias was evaluated using funnel plot and Egger's regression test. No significant publication bias was seen in case of COVID-19 versus control, Non-severe versus severe and mechanical ventilation required versus did not require. Significant publication bias was detected in severe to critical versus mild to moderate. Details showed in Supp. Fig. 5.
4. Discussion
In our study, high ferritin level was observed among COVID-19 patients compared to controls [control vs. COVID-19, SMD −0.889 (95% C.I. −1.201, −0.577), I2 = 85%]. COVID-19 patients with severe to critical disease had higher ferritin level compared to patients with mild to moderate disease [SMD 0.882 (0.738, 1.026), I2 = 85%]. Non-survivors had higher serum ferritin level compared to survivors [SMD 0.992 (0.672, 1.172), I2 = 92.33%]. Patients requiring ICU [SMD 0.674 (0.515 to 0.833), I2 = 80%] and mechanical ventilation [SMD 0.430 (0.258, 0.602), I2 = 32%] had higher serum ferritin levels compared to those who didn't require ICU and did not require mechanical ventilation. Higher level of ferritin was also noted among COVID-19 patients with kidney involvement; however, in case of association between ferritin level and COVID-19 associated liver injury, data was heterogeneous. Patients without COVID-19 related thrombotic complications had lower serum ferritin levels compared to patients showing thrombotic complications. These findings highlight the importance of serum ferritin as a biomarker of severity in COVID-19.
The major limitation of our study is that high heterogeneity was seen among the included studies for many of the comparisons. However, we have explored the possible causes of this high heterogeneity using predefined sub-group analysis and meta-regression analysis. Metaregression was conducted in case there were 10 or more studies for a possible confounding variable. High heterogeneity was seen in many of the comparisons (COVID-19 negative versus COVID-19 positive, severe to critical disease vs. mild to moderate disease, non-survivor vs. survivor and patients requiring ICU versus patients who did not require ICU). Subgroup analyses on the basis of predefined subgroups couldn't pinpoint the etiology of high heterogeneity in any of the comparisons. Metaregression was carried out in case of two comparisons: severe and critical versus mild to moderate disease and non-survivor versus survivor. In metaregression for the severe to critical versus mild and moderate comparison, high heterogeneity observed could be attributed to difference between the two severity categories in terms of “mean age”, and “percentage of population with concomitant co-morbidities”. In metaregression for the non-survivor versus survivor comparison, high heterogeneity observed could be attributed to difference in “mean age” and “percentage of male sex” between the two groups, which indicate that age and gender can also influence serum ferritin levels significantly.
5. Conclusion
To conclude, high serum ferritin level was found to be associated with more severe disease and negative/poor outcome in COVID-19. Thus serum ferritin level can serve as an important predictive biomarker in COVID-19 management and in triage. However, in presence of other co-morbid conditions/disease, serum ferritin level needs to be interpreted cautiously.
Author contribution statement
KK, HK, PS and AB had written the manuscript; KK, MP, SK and MK did the literature search; KK, PS, BM designed the research, KK, PS, HK, MP and AB analyzed data and rest all the other authors reviewed and finalized the manuscript.
Funding
Nil.
Declaration of competing interest
Nil.
Acknowledgments
Acknowledgement
Nil.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2021.09.023.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
Supplementary material 3
References
- 1.Kernan K.F., Carcillo J.A. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plays M., Müller S., Rodriguez R. Chemistry and biology of ferritin. Metallomics. 2021:mfab021. doi: 10.1093/mtomcs/mfab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang B., Thompson M.S., Adkins K.M. Characteristics of the iron-responsive element (IRE) stems in the untranslated regions of animal mRNAs. Open Biochem J. 2021;15 doi: 10.2174/1874091X02115010026. [DOI] [Google Scholar]
- 4.Edeas M., Saleh J., Peyssonnaux C. Iron: innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colafrancesco S., Alessandri C., Conti F., Priori R. COVID-19 gone bad: a new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev. 2020;19:102573. doi: 10.1016/j.autrev.2020.102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drakesmith H., Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6:541–552. doi: 10.1038/nrmicro1930. [DOI] [PubMed] [Google Scholar]
- 7.Liu W., Zhang S., Nekhai S., Liu S. Depriving iron supply to the virus represents a promising adjuvant therapeutic against viral survival. Curr Clin Microbiol Rep. 2020:1–7. doi: 10.1007/s40588-020-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pretorius E., Kell D.B. Diagnostic morphology: biophysical indicators for iron-driven inflammatory diseases. Integr Biol. 2014;6:486–510. doi: 10.1039/c4ib00025k. [DOI] [PubMed] [Google Scholar]
- 9.Lipinski B., Pretorius E., Oberholzer H.M., Van Der Spuy W.J. Iron enhances generation of fibrin fibers in human blood: implications for pathogenesis of stroke. Microsc Res Tech. 2012;75:1185–1190. doi: 10.1002/jemt.22047. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Cao W., Xiao M., Li Y.J., Yang Y., Zhao J., et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41 doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PubMed] [Google Scholar]
- 11.Mazzeffi M.A., Chow J.H., Tanaka K. COVID-19 associated hypercoagulability: manifestations, mechanisms, and management. Shock. 2021;55:465–471. doi: 10.1097/SHK.0000000000001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeMartino A.W., Rose J.J., Amdahl M.B., Dent M.R., Shah F.A., Bain W., et al. No evidence of hemoglobin damage by SARS-CoV-2 infection. Haematologica. 2020;105:2769–2773. doi: 10.3324/haematol.2020.264267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahalmani V., Sarma P., Prakash A., Medhi B. Role of iron chelators in mucormycosis. Indian J Pharm. 2021;53:261. doi: 10.4103/ijp.ijp_604_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulchandani R., Lyngdoh T., Kakkar A.K. Deciphering the COVID-19 cytokine storm: systematic review and meta-analysis. Eur J Clin Invest. 2021;51 doi: 10.1111/eci.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Z., Long F., Yang Y., Chen X., Xu L., Yang M. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng L., Li H., Li L., Liu C., Yan S., Chen H., et al. Ferritin in the coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Lab Anal. 2020;34 doi: 10.1002/jcla.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.JPT Higgins, Thomas J., Chandler J., Cumpston M., Li T., Page M.J., et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Handbook for Systematic Reviews of Interventions Version 62. 2021. https://training.cochrane.org/handbook
- 20.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arevalo-Rodriguez I., Buitrago-Garcia D., Simancas-Racines D., Zambrano-Achig P., Campo R.D., Ciapponi A., et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middleton P., Perez-Guzman P.N., Cheng A., Kumar N., Kont M.D., Daunt A., et al. Characteristics and outcomes of clinically diagnosed RT-PCR swab negative COVID-19: a retrospective cohort study. Sci Rep. 2021;11:2455. doi: 10.1038/s41598-021-81930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Case definition for coronavirus disease 2019 (COVID-19), as of 3 December 2020. European Centre for Disease Prevention and Control n.d. https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition (accessed August 22, 2021).
- 24.World Health Organization . World Health Organization; 2020. Clinical management of COVID-19: interim guidance, 27 May 2020. [Google Scholar]
- 25.Sarma P., Bhattacharyya A., Kaur H., Prajapat M., Prakash A., Kumar S., et al. Efficacy and safety of steroid therapy in COVID-19: a rapid systematic review and meta-analysis. Indian J Pharm. 2020;52:535. doi: 10.4103/ijp.ijp_1146_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. He Ottawa Hospital Research Institute; 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed September 26, 2021) [Google Scholar]
- 27.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 28.RevMan 5 (Version 5.4.1) 2021. https://TrainingCochraneOrghttps://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download (accessed September 26, 2021)
- 29.Bhattacharyya A., Kumar S., Sarma P., Kaur H., Prajapat M., Shekhar N., et al. Safety and efficacy of lopinavir/ritonavir combination in COVID-19: a systematic review, meta-analysis, and meta-regression analysis. Indian J Pharm. 2020;52:313–323. doi: 10.4103/ijp.IJP_627_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cullis J.O., Fitzsimons E.J., Griffiths W.J., Tsochatzis E., Thomas D.W., British Society for Haematology Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331–340. doi: 10.1111/bjh.15166. [DOI] [PubMed] [Google Scholar]
- 32.Maneeton N., Maneeton B., Putthisri S., Woottiluk P., Narkpongphun A., Srisurapanont M. Risperidone for children and adolescents with autism spectrum disorder: a systematic review. Neuropsychiatr Dis Treat. 2018;14:1811–1820. doi: 10.2147/NDT.S151802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer A.J., Morley E.J., Meyers K., Fernandes R., Rowe A.L., Viccellio P., et al. Cohort of four thousand four hundred four persons under investigation for COVID-19 in a New York hospital and predictors of ICU care and ventilation. Ann Emerg Med. 2020 doi: 10.1016/j.annemergmed.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermans J.J.R., Groen J., Zwets E., Boxma-De Klerk B.M., Van Werkhoven J.M., Ong D.S.Y., et al. Chest CT for triage during COVID-19 on the emergency department: myth or truth? Emerg Radiol. 2020:1–11. doi: 10.1007/s10140-020-01821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benussi A., Pilotto A., Premi E., Libri I., Giunta M., Agosti C., et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020;95:e910–e920. doi: 10.1212/WNL.0000000000009848. [DOI] [PubMed] [Google Scholar]
- 36.Hernández-Fernández F., Sandoval Valencia H., Barbella-Aponte R.A., Collado-Jiménez R., Ayo-Martín Ó., Barrena C., et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143:3089–3103. doi: 10.1093/brain/awaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phipps M.M., Barraza L.H., LaSota E.D., Sobieszczyk M.E., Pereira M.R., Zheng E.X., et al. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large U.S. cohort. Hepatology. 2020;72:807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramachandran P., Perisetti A., Gajendran M., Chakraborti A., Narh J.T., Goyal H. Increased serum aminotransferase activity and clinical outcomes in coronavirus disease 2019. J Clin Exp Hepatol. 2020 doi: 10.1016/j.jceh.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Da B.L., Kushner T., Halabi M.E., Paka P., Khalid M., Uberoi A., et al. Liver injury in patients hospitalized with coronavirus disease 2019 correlates with hyperinflammatory response and elevated interleukin-6. Hepatol Commun. 2021;5:177–188. doi: 10.1002/hep4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishra K., Naffouj S., Gorgis S., Ibrahim H., Gill S., Fadel R., et al. Liver injury as a surrogate for inflammation and predictor of outcomes in COVID-19. Hepatol Commun. 2021;5:24–32. doi: 10.1002/hep4.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang D., Zhu Z., Bi J., Feng D., Zhang L., Liu H., et al. Clinical characteristics and risk factors in coronavirus disease 2019 patients with liver injury. Med Sci Monit. 2020;26:e928849. doi: 10.12659/MSM.928849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohamed M.M.B., Lukitsch I., Torres-Ortiz A.E., Walker J.B., Varghese V., Hernandez-Arroyo C.F., et al. Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans. Kidney. 2020;360(1):614–622. doi: 10.34067/KID.0002652020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azam T.U., Shadid H.R., Blakely P., O’Hayer P., Berlin H., Pan M., et al. Soluble urokinase receptor (SuPAR) in COVID-19-related AKI. J Am Soc Nephrol. 2020;31:2725–2735. doi: 10.1681/ASN.2020060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J.R., Silberzweig J., Akchurin O., Choi M.E., Srivatana V., Lin J., et al. Characteristics of acute kidney injury in hospitalized COVID-19 patients in an urban academic medical center. CJASN. 2021;16:284–286. doi: 10.2215/CJN.07440520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansrivijit P., Gadhiya K.P., Gangireddy M., Goldman J.D. Risk factors, clinical characteristics, and prognosis of acute kidney injury in hospitalized COVID-19 patients: a retrospective cohort study. Medicines. 2021;8:4. doi: 10.3390/medicines8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng S., Wang H.-Y., Sun X., Li P., Ye Z., Li Q., et al. Early versus late acute kidney injury among patients with COVID-19—a multicenter study from Wuhan, China. Nephrol Dial Transplant. 2020;35:2095–2102. doi: 10.1093/ndt/gfaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3