Abstract
Purpose
Hyperalgesia and bladder overactivity are two main symptoms of interstitial cystitis/bladder pain syndrome (IC/BPS). Cannabinoid receptors participate in the modulation of pain and bladder function. GPR18, a member of the cannabinoid receptor family, also participates in the regulation of pain and bladder function, but its underlying mechanisms are unknown. In this work, we sought to study the role of GPR18 in IC/BPS.
Methods
A rat model of IC/BPS was established with cyclophosphamide (CYP). Paw withdrawal threshold (PWT) measurement and cystometry were used to evaluate pain and bladder function, respectively. RT-PCR, Western blotting and immunofluorescence were used to assess the expression and distribution of GPR18. The role of GPR18 in pain and bladder function was studied by intrathecal injection of resolvin D2 (RvD2, a GPR18 agonist) and O-1918 (a GPR18 antagonist). Calcium imaging was used to study the relationship between GPR18 and TRPV1.
Results
A rat model of IC/BPS, which exhibited a decreased PWT and micturition interval, was successfully established with CYP. The mRNA and protein expression of GPR18 was reduced in the bladder and dorsal root ganglia (DRG) in rats with CYP-induced cystitis. Intrathecal injection of RvD2 increased the PWT and micturition interval. However, O-1918 blocked the therapeutic effect of RvD2. GPR18 was present in bladder afferent nerves and colocalized with TRPV1 in DRG, and RvD2 decreased capsaicin-induced calcium influx in DRG.
Conclusion
Activation of GPR18 by RvD2 alleviated hyperalgesia and improved bladder function, possibly by inhibiting TRPV1 in rats with CYP-induced cystitis.
Keywords: interstitial cystitis/bladder pain syndrome, GPR18, TRPV1, hyperalgesia, bladder overactivity, resolvin D2, O-1918
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is defined as pelvic pain associated with bladder and lower urinary tract symptoms when there is no clear cause.1 The prevalence of IC/BPS is 0.25% to 1.22% in men and 0.83% to 2.71% in women.2 Millions of dollars are spent on the treatment of IC/BPS every year, but the therapeutic effect of available treatments is not satisfactory.3,4 There are many theories about the pathogenesis of IC/BPS, such as the myogenic hypothesis, afferent hypothesis, and inflammatory factor hypothesis, but the exact mechanism remains unclear.5–7
The endogenous cannabinoid system (ECS) is composed of endocannabinoids, cannabinoid receptors, and related enzymes.8 A great number of studies have proven that the ESC participates in the modulation of pain and bladder function. Treatment with exogenous cannabinoids relieves pain in patients with diabetic neuropathy disease, neuropathic pain, and multiple sclerosis.9–11 Intravesical instillation of WIN 552122, a selective cannabinoid receptor agonist, improves detrusor overactivity.12 Further studies have shown that cannabinoid receptors are involved in the regulation of pain and bladder function by inhibiting TRPV1.13,14 GPR18, a cannabinoid receptor in the ECS, also participates in the modulation of pain. Resolvin D2 (RvD2), a specific GPR18 agonist, alleviates cancer pain in oral squamous cell carcinoma.15 Likewise, activating GPR18 with RvD2 can inhibit TRPV1 in inflammatory bowel disease.16
However, the role of GPR18 in IC/BPS is still unclear. In this study, we established an IC/BPS rat model to explore the role of GPR18 in pain and bladder function.
Materials and Methods
Animals and Model
Female Sprague-Dawley (SD) rats (200–250 g) were purchased from the Experimental Animal Center of Army Medical University (Chongqing, China). The rats were housed at a suitable temperature (22±2 °C) and humidity (40–60%) on a 12 h light/dark cycle and given free access to food and water. The experimental procedures were approved by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cyclophosphamide (CYP, Merck, USA) was dissolved in saline. CYP (200 mg/kg) was injected intraperitoneally to establish an IC/BPS model as described in a previous report.17 The rats in the control (Con) group were intraperitoneally injected with saline.
Tissue Specimens
The bladder and L6-S1 dorsal root ganglia (DRG) were collected 4, 24, and 48 h after intraperitoneal injection of CYP or saline and fixed in 4% paraformaldehyde for 1 day. The bladder and DRG were cut into paraffin sections and frozen sections, respectively. Then, the sections were subjected to HE staining and immunofluorescence staining.
Drug Administration
To analyse the role of GPR18 in CYP-induced cystitis, we used a GPR18 agonist, RvD2 (MCE, USA), and a GPR18 antagonist, O-1918 (Tocris, UK). RvD2 was dissolved in sterile PBS while minimizing exposure to light. RvD2 (10 ng, 100 ng) or vehicle (2% ethanol in PBS) was injected intrathecally as described in previous studies.18,19 Before using O-1918, the methyl acetate was evaporated under a gentle stream of nitrogen, and vehicle was immediately added. The final concentration of O-1918 was 25 mM. Spinal puncture was performed between the L5 and L6 levels using a PE-10 catheter for intrathecal injection of the reagent (30 µL).
Paw Withdrawal Threshold
Measurement of the paw withdrawal threshold (PWT) is a good method for assessing pain.17,20 The PWT was assessed by using the “up-down” method with some modifications.21 The rats were acclimatized to the cage for 30 min before testing. Starting with the 8 g hair, a series of Von Frey hairs (2, 4, 6, 8, 10, 15, 26, 60 g) were applied to the right hindpaw. Lifting or licking the hindpaw was considered a positive response. To rule out differences between individuals, we used the PWT at 0 h as the basal PWT.
Cystometry
Rats were anaesthetized by intraperitoneal injection of urethane. One end of a PE-10 catheter was transurethrally inserted into the bladder, and the other end was connected to a tee that was connected with a syringe pump (SN-50F6, SINOMDT, China) and a pressure sensor (RM6240C, Chengdu Instrument Factory, China). Saline (8 mL/h) was continuously infused into the bladder through the catheter. After cystometric measurements were stable, we began to record and analyze the basal bladder pressure, maximum bladder pressure, and micturition interval.
Quantitative Real-Time PCR
Using TRIzol reagent (9108, Takara, Japan), total RNA was extracted from the bladder and L6-S1 DRG. After determining the RNA concentration and purity by using a spectrophotometer (Micro Drop, Bio-DL, USA), the RNA was reverse transcribed into cDNA using reverse transcription reagent (RR047A, Takara, Japan). Finally, real-time PCR analysis was performed on a real-time PCR instrument (4376600, Thermo Fisher, USA) using fluorescent reagent (208054, QIAGEN, Germany). The primer sequences were as follows: GPR18: 5ʹ-CATCTCAGCAACCCTCCAAC-3ʹ and 5ʹ-CCATGGTACGTAGAAACTCCTG-3ʹ; TRPV1: 5ʹ-CTCCAAGGCACTTGCTCCAT-3ʹ and 5ʹ-GTGGCTTGCAGTTAGGGTCT-3ʹ. A GAPDH primer was purchased from Sangon Biotech (B661204, China).
Western Blotting
Bladders were collected 4, 24, and 48 h after injection of CYP or saline. Total protein was extracted from the bladders using lysis buffer (P0013B, Beyotime, China). The proteins were separated by 10% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with blocking buffer (P0252, Beyotime, China) for 1 h at room temperature (RT) and then incubated with the following primary antibodies overnight at 4°C: GPR18 (1:2000, ab174835, Abcam, England), TRPV1 (1:1000, ab203103, Abcam, England) and GAPDH (1:2000, 60004-1-Ig, Proteintech, USA). Then, the membranes were washed with TBST and incubated with secondary antibodies (1:2000, G-21234 or G21040, Thermo Fisher, USA) for 1 h at RT. Finally, the target protein bands were detected using an ECL chemiluminescence kit (32132X3, Thermo Fisher, USA). To analyse the protein band density, ImageJ software was used.
Immunofluorescence
Before staining, bladder paraffin sections were dewaxed and subjected to antigen retrieval with antigen repair solution (P0081, Beyotime, China). The sections were blocked with blocking buffer (P0260, Beyotime, China) for 15 min and then incubated overnight with the following primary antibodies at 4°C: GPR18 (1:200, PA5-23218, Thermo Fisher, USA), PGP9.5 (1:1000, PA1-10011, Thermo Fisher, USA), and TRPV1 (1:200, PA5-111831, Thermo Fisher, USA). After being washed with PBS, the sections were incubated with secondary antibodies for 2 h at RT. Finally, the sections were viewed using a SlideView VS200 slide scanner (Olympus, Japan), and the mean fluorescence intensity was analysed.
Primary DRG Culture
L6-S1 DRGs were cultured as described previously.22 After the rats were anaesthetized, the L6-S1 DRGs were quickly removed and incubated with digestive enzymes, ie, 2 mg/mL collagenase II (Absin, China) and 2 mg/mL dispase (Absin, China), in Hank’s balanced solution (HBSS, with calcium and magnesium) at 37°C for 45 min. DMEM/F12 (Zhong Qiao Xin Zhou, China) was used to stop the digestion, and neurons were seeded in Petri dishes and cultured in DMEM/F12.
Calcium Imaging
After being cultured for 12 h, neurons were incubated with 2 μM Fluo-4 AM (Beyotime, China) at 37°C for 20 min, washed with HBSS (without calcium and magnesium) 3 times and incubated for 20 min. A laser confocal microscope (Spin SR10, Olympus, Japan) was used to detect the change in calcium fluorescence intensity in real time. The basal fluorescence intensity of calcium (F0) was recorded, and then the fluorescence intensity of calcium influx induced by 1 μM capsaicin (F1). To study the effect of GPR18 on TRPV1, neurons were incubated with 10 nM RvD2 and 30 μM O-1918, as described by Perna.16 The F1/F0 ratio was calculated as the change in the intracellular calcium concentration.
Statistical Analysis
All the data are expressed as the mean±standard deviation. Statistical analysis was performed using Student’s t-test, paired-samples t-test, one-way ANOVA, or two-way ANOVA followed by the Newman-Keuls post hoc test with a significance threshold of P < 0.05.
Results
A Rat Model of IC/BPS Was Successfully Established with CYP
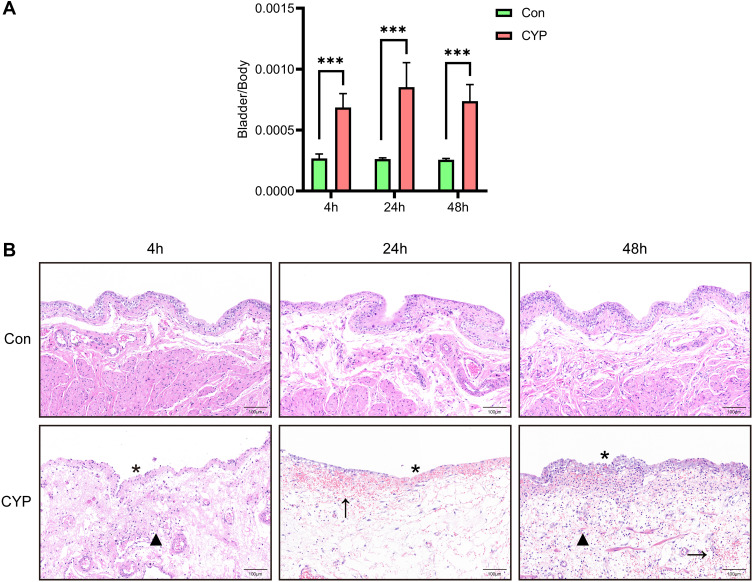
According to the method described by Boucher,17 we successfully constructed an IC/BPS model. Measurement of the bladder weight to body weight ratio and HE staining were conducted 4, 24, and 48 h after intraperitoneal injection of CYP or saline. The bladder weight to body weight ratio increased significantly and was the highest at 24 h in the CYP group (Figure 1A). The CYP-treated bladders exhibited much thicker submucosal oedema than the control bladders (Figure 1B). Microscopically, submucosal haemorrhaging, epithelial ulceration, and infiltration of inflammatory cells were evident in the CYP group (Figure 1B).
Figure 1.
A rat model of IC/BPS was successfully established by administration of CYP. Analysis was performed 4, 24, and 48 h after intraperitoneal injection of saline or CYP. (A) The bladder weight to body weight ratio (n=6). (B) HE staining of bladder sections from saline- and CYP-treated rats. The stars indicate epithelial ulceration, the arrows indicate submucosal haemorrhaging, and the triangles indicate infiltration of inflammatory cells. Student's t-test was for statistical analysis. ***P<0.001.
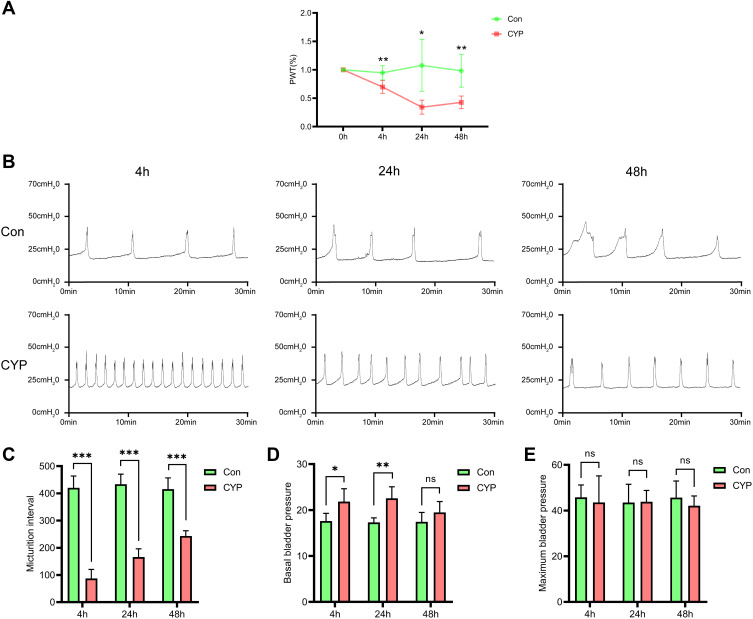
The PWT and Micturition Interval Were Decreased in the CYP Group
A reduction in the PWT was observed as early as 4 h after injection of CYP, and the PWT was the lowest at 24 h in the CYP group (0.95 vs 0.70, 1.08 vs 0.34, and 0.98 vs 0.43; P<0.01, P<0.05 and P<0.01, respectively; Figure 2A). Cystometry revealed that overactive bladder was successfully induced by CYP (Figure 2B). The micturition interval was decreased at 4, 24, and 48 h in the CYP group (420.42 vs 87.26 s, 433.47 vs 165.85 s and 415.67 vs 243.26 s; P<0.001, 0.001, and 0.001, respectively; Figure 2C). The basal bladder pressure was only increased at 4 and 24 h (17.60 vs 21.84 and 17.31 vs 22.55; P<0.05 and 0.01, respectively; Figure 2D), and the maximum bladder pressure was not changed at any time point (Figure 2E).
Figure 2.
CYP successfully induced hyperalgesia and bladder overactivity 4, 24, and 48 h after intraperitoneal injection of saline or CYP. (A) The change in the PWT (n=6). The basal PWT at 0 h was defined as 100%. (B) Representative cystometrogram traces and (C–E) statistical analysis of the micturition interval, basal bladder pressure, and maximum bladder pressure (n=5). Student's t-test was used for statistical analysis. *P<0.05; **P<0.01; ***P<0.001; ns, no significance.
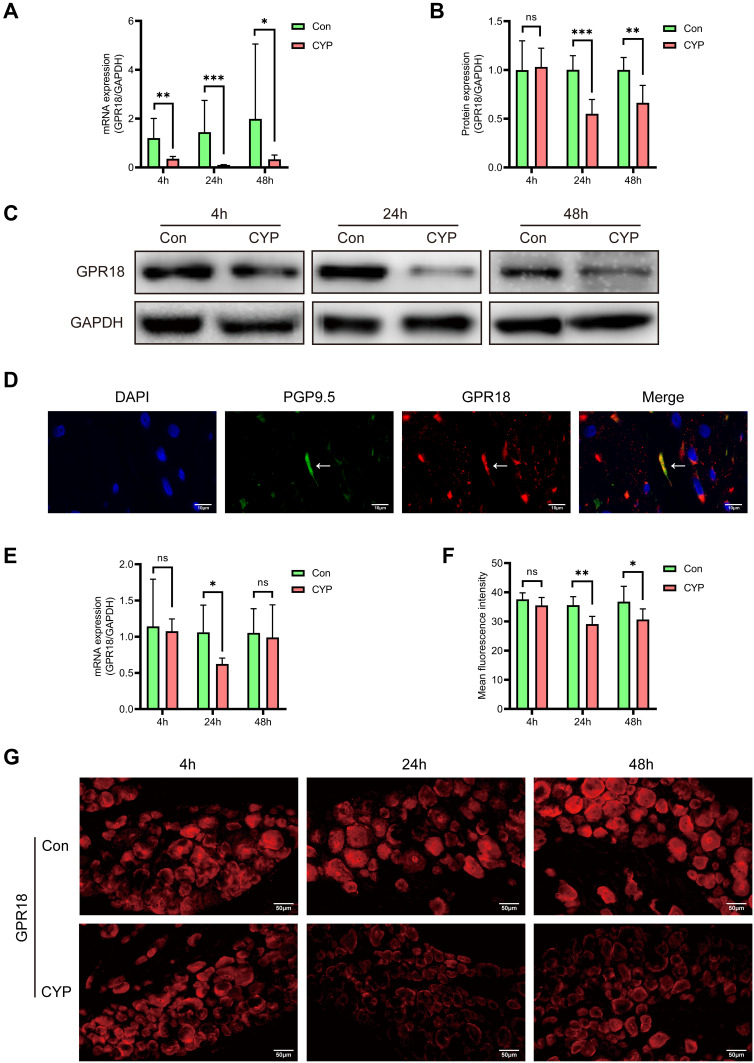
The Expression of GPR18 Was Downregulated in Rats with CYP-Induced Cystitis
To investigate the role of GPR18, we first studied the expression of GPR18 in the bladder. The mRNA expression of GPR18 was decreased at all times in the CYP group (1.20 vs 0.35, 1.44 vs 0.11 and 1.98 vs 0.33; P<0.01, 0.001 and 0.05, respectively; Figure 3A). Similarly, the protein level of GPR18 was reduced at 24 and 48 h (1.00 vs 0.55 and 1.00 vs 0.66, P<0.001 and 0.01, respectively, Figure 3B and C) but not at 4 h in the CYP group.
Figure 3.
The expression of GPR18 was decreased in the bladders and DRGs of rats with CYP-induced cystitis 4, 24, and 48 h after intraperitoneal injection of saline or CYP. (A) Analysis of the mRNA expression of GPR18 in the bladders (n=6). (B and C) Analysis of the protein expression of GPR18 and representative Western blotting images of GPR18 in the bladder (n=6). (D) Analysis of the coexpression of GPR18 (red) and PGP9.5 (green) in normal bladder sections using immunofluorescence. (E) Analysis of the mRNA expression of GPR18 in the L6-S1 DRGs (n=6). (F and G) Analysis of the expression and distribution of GPR18 in the L6-S1 DRGs using immunofluorescence (n=6). The arrows indicate the afferent nerves that supply the bladder. Student's t-test was used for statistical analysis. *P<0.05; **P<0.01; ***P<0.001; ns, no significance.
Some studies have proven that GPR18 is expressed in the central and peripheral nervous systems;16,18 thus, we decided to study the distribution of GPR18 in the bladder afferent pathway. Immunofluorescence analysis of PGP9.5, a nerve fibre marker, was used to confirm the existence of GPR18 in the bladder afferent pathway in normal bladder specimens. PGP9.5 and GPR18 were coexpressed in the bladder afferent nerve in normal bladders (Figure 3D). Then, the expression of GPR18 in the L6-S1 DRGs was assessed. The mRNA expression of GPR18 was reduced at 24 h (1.06 vs 0.63; P<0.05; Figure 3E) but not at 4 or 48 h in the CYP group. Immunofluorescence showed that GPR18 was expressed in neurons of all sizes. The fluorescence intensity of GPR18 was decreased at 24 and 48 h (35.57 vs 29.10 and 36.78 vs 30.67; P<0.01 and 0.05, respectively) but not at 4 h (Figure 3F and G).
The expression of GPR18 was decreased in both the bladders and DRGs, suggesting that GPR18 might play an important role in CYP-induced cystitis.
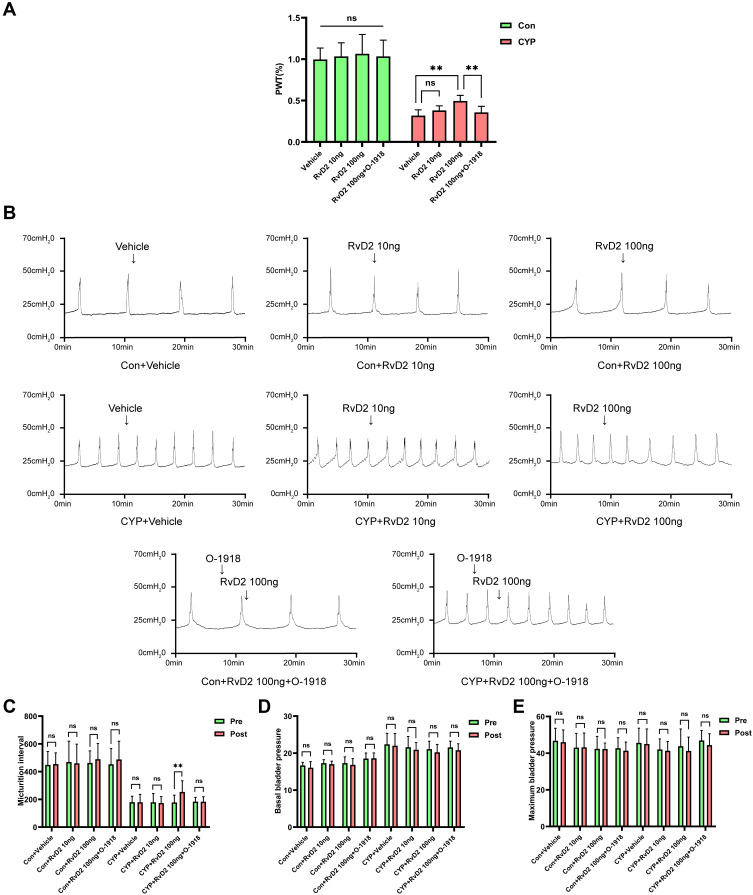
RvD2 Alleviated Hyperalgesia and Improved Bladder Overactivity
Because the expression of GPR18 was the lowest 24 h after the injection of CYP, the 24 h CYP-induced cystitis model was used to study the role of GPR18. The PWT was significantly increased in the 100 ng RvD2-treated group compared with the CYP+vehicle group (0.32 vs 0.49; P<0.01), but not in the 10 ng RvD2-treated group (Figure 4A). However, O-1918 blocked the effect of RvD2 in increasing the PWT (0.36 vs 0.49; P<0.01; Figure 4A). In addition, we studied the effect of RvD2 on the basal PWT. The basal PWT was not affected in the 10 ng RvD2-treated group or 100 ng RvD2-treated group compared with the Con+vehicle group (Figure 4A). Then, the effect of RvD2 on bladder function was studied. Intrathecal injection of 100 ng RvD2 increased the micturition interval in the CYP group (178.31 vs 253.36; P<0.01), but 10 ng RvD2 and vehicle did not influence the micturition interval (Figure 4B and C). Likewise, O-1918 blocked the effect of RvD2 in increasing the micturition interval (183.58 vs 183.43; P>0.05; Figure 4B and C). RvD2 (10 and 100 ng) did not influence the normal micturition interval in the Con group (Figure 4B and C). Moreover, basal bladder pressure and maximum bladder pressure were not influenced by any treatments (Figure 4B, D and E).
Figure 4.
Intrathecal injection of RvD2 increased the PWT and micturition interval 24 h after intraperitoneal injection of CYP. (A) The effects of intrathecal injection of different drugs on the PWT (n=6). (B) Representative cystometrogram traces and (C–E) statistical analysis of the micturition interval, basal bladder pressure, and maximum bladder pressure before and after intrathecal injection of different drugs (n=5). Pre, before intrathecal injection of drugs; Post, after intrathecal injection of drugs. The arrows indicate the timing of intrathecal drug injection. One-way ANOVA and paired-samples t-test were used for statistical analysis, **P<0.01; ns, no significance.
The Expression of TRPV1 Was Increased in Rats with CYP-Induced Cystitis
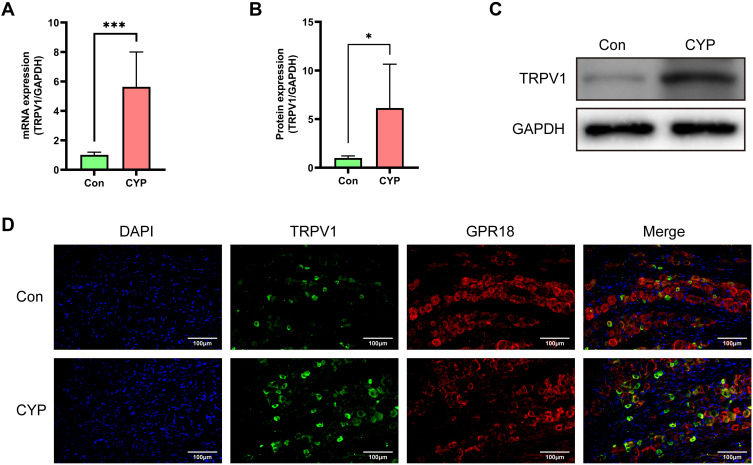
TRPV1 has been proven to be a therapeutic target for IC/BPS and is coexpressed with cannabinoid receptors; thus, we speculated that GPR18 is coexpressed with TRPV1.22–24 In rats with CYP-induced cystitis, the mRNA and protein expression of TRPV1 was increased (1.01 vs 5.63 and 1.00 vs 6.13; P<0.001 and 0.05; Figure 5A–C). Immunofluorescence showed that GPR18 and TRPV1 were coexpressed in small- and medium-sized neurons (Figure 5D). These results suggested that GPR18 might function through TRPV1.
Figure 5.
The expression of TRPV1 was increased in the CYP group, and TRPV1 colocalized with GPR18 in the L6-S1 DRGs 24 h after intraperitoneal injection of saline or CYP. (A) Analysis of the mRNA expression of TRPV1 in the bladders (n=6). (B and C) Analysis of the protein expression of TRPV1 and representative Western blotting image of TRPV1 in the bladders (n=6). (D) The colocalization of TRPV1 and GPR18 in the L6-S1 DRGs. Student's t-test was used for statistical analysis. *P<0.05; ***P<0.001.
RvD2 Inhibited the Sensitivity of TRPV1 in the DRGs of the CYP Group
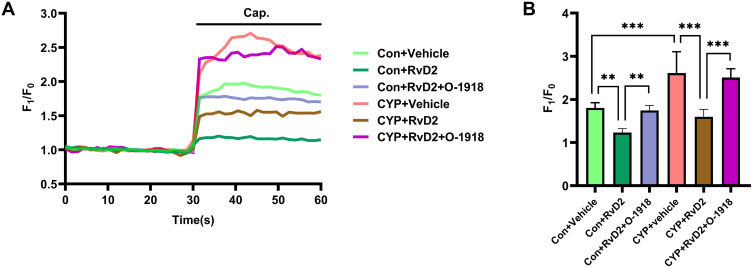
To investigate whether GPR18 participates in modulating the sensitivity of TRPV1 in the DRGs, calcium imaging was conducted. Capsaicin induced a stronger influx of calcium in the CYP group than in the Con group (1.80 vs 2.61; P<0.001; Figure 6A and B). In both the Con and CYP groups, RvD2 inhibited capsaicin-induced calcium influx (1.80 vs 1.23 and 2.61 vs 1.60; P<0.01 and 0.001, respectively; Figure 6A and B). However, capsaicin-induced calcium influx was not inhibited in those groups treated with O-1918 and RvD2 (1.75 vs 1.23 and 2.51 vs 1.60; P<0.01 and 0.001, respectively; Figure 6A and B). These results indicated that the activation of GPR18 by RvD2 can inhibit the sensitivity of TRPV1 in the L6-S1 DRGs in rats with CYP-induced cystitis.
Figure 6.
RvD2 inhibited the sensitivity of TRPV1 in the DRGs of the CYP group. (A) Representative curve of the calcium fluorescence intensity under different treatments and (B) statistical analysis of the changes in calcium fluorescence intensity (n=6). Cap., capsaicin stimulation. Two-way ANOVA followed by the Newman-Keuls post hoc test was used for statistical analysis. **P<0.01; ***P<0.001.
Discussion
In this research, we found that GPR18 was involved in hyperalgesia and overactive bladder. The PWT was decreased, and bladder function was overactive 4, 24, and 48 h after the induction of cystitis by CYP. The expression of GPR18 was significantly reduced in the bladders and DRGs of the CYP group. Intrathecal injection of RvD2, a specific GPR18 agonist, relieved pain and alleviated bladder overactivity. Activation of GPR18 by RvD2 inhibited capsaicin-induced activation of TRPV1. These results showed that the activation of GPR18 by RvD2 can relieve pain and improve bladder function, possibly through inhibition of TRPV1.
Previous studies have found that cannabis can improve bladder function in patients with multiple sclerosis, but the exact mechanisms are unknown.25 The ECS is composed of cannabinoid receptors, endocannabinoids and enzymes that regulate endocannabinoid biosynthesis and degradation.8 Cannabinoid receptors include CB1, CB2, GPR18 and GPR55. Many reports have proven that CB1 and CB2 are expressed in bladder afferent nerve fibres, the L6-S1 DRGs, and the L6-S1 spinal cord.26,27 Likewise, GPR18 is distributed in the central nervous system, including the cerebellum and spinal cord, as well as in peripheral nervous system, including the DRGs.18 In our experiment, we also found that GPR18, which was colocalized with PGP9.5, was expressed in bladder afferent nerve fibres and in the L6-S1 DRGs. In patients with CYP-induced cystitis and detrusor overactivity, the expression of CB1 and CB2 is increased.12,26 Conversely, we found that the expression of GPR18 was decreased in the bladders and DRGs of rats with CYP-induced cystitis. These results suggest that GPR18 might be a target for the treatment of CYP-induced cystitis.
Pelvic pain and bladder overactivity are the two main clinical symptoms of IC/BPS.28 Systemic injection of cannabinoid receptor agonists and cannabinoid analogues alleviate referred pain associated with cystitis.29–31 Likewise, intrathecal injection of cannabinoid receptor agonists attenuates cancer pain,32,33 and intravesical infusion of CB1 and CB2 agonists alleviates detrusor overactivity.12 GPR18 is also involved in the regulation of pain and bladder function. PSB-KK-1415, a GPR18 agonist, has been shown to exert antinociceptive effects in pain models.34 Plantar injection of NAGly, a GPR18 agonist, mitigates formalin-induced inflammatory pain, and systemic injection of O-1602, a nonselective GPR18/GPR55 agonist, ameliorates detrusor overactivity.35–38 Additionally, we found that CYP significantly decreased the mechanical pain threshold and that intrathecal injection of RvD2, a selective endogenous GPR18 agonist, alleviated CYP-induced hyperpathia. We observed that the micturition interval was decreased and the basal bladder pressure was increased in the CYP group and that intrathecal injection of RvD2 increased the micturition interval. O-1918, an antagonist of GPR18, reversed the effect of RvD2 on the PWT and micturition interval.
The urination and storage function of the bladder is regulated by the nervous system.39 Recent findings revealed that bladder afferent nerve hyperexcitability was involved in the pathological mechanisms of IC/BPS.40,41 Bladder afferent nerve fibres consist of Aδ fibres and C fibres, and the cell bodies of bladder afferent neurons reside in the DRGs.42 Aδ fibres respond to the normal contraction and diastolic function of the bladder. C fibres mainly respond to noxious stimuli, such as capsaicin, low pH, and inflammatory factors. Aδ fibres participates in the regulation of normal bladder function, while C fibres only participate in the modulation of bladder function under pathological conditions.43 In IC/BPS and cystitis, the density of bladder afferent nerve fibres increases.44,45 In addition, the excitability of bladder C afferent nerves is enhanced, as indicated by increased afferent firing, a decreased activating threshold and an increased evoked action potential area.46–48
It has been proven that the sensitization of TRPV1 is a cause of bladder afferent nerve hyperexcitability.22 TRPV1, a calcium ion channel that is mainly expressed in C fibres, participates in mechanosensitive and nociceptive conduction.49 Clinical evidence has shown that elevation of the number of TRPV1-positive nerve fibres is correlated with the severity of pain and urgency.44 In CYP-induced cystitis, TRPV1 in the bladder afferent nerve become sensitized. In CYP-induced cystitis, a higher current density can be induced by capsaicin in the afferent nerve, and the afferent nerve can be activated by heat at a lower temperature than in the normal bladder.50 Some inflammatory mediators, such as nerve growth factor and histamine, can increase the expression of TRPV1 and sensitize TRPV1, causing TRPV1 to contribute to bladder afferent nerve hyperexcitability.51,52 Clinically, intravesical instillation of resiniferatoxin and capsaicin has been used to treat bladder overactivity because resiniferatoxin and capsaicin can desensitize TRPV1 and reduce the number of TRPV1-positive nerve fibres in the bladder.53,54 Knockout or drug-mediated blockade of TRPV1 also alleviates bladder overactivity and pain.55,56 These studies suggest that TRPV1 is an important target for the treatment of IC/BPS.
Electrophysiological studies have found that cannabin receptor agonists inhibit the activity of bladder afferent nerve fibres.57–59 URB937, an inhibitor of endocannabinoid degradation, reduces the hyperactivity of bladder afferent nerve fibres and improves bladder function.60 As mentioned above, CB1 and CB2 are distributed in bladder afferent nerve fibres and the DRGs. Further research has revealed that CB1 and CB2 are coexpressed with TRPV1 in bladder afferent nerve fibres and the DRGs.24,58,61 Researchers have found that CB1 and CB2 inhibit the activity of afferent fibres induced by capsaicin.14,62 These results indicate that cannabinoid receptors block the activity of afferent nerves by inhibiting TRPV1.14 Thus, we hypothesized that GPR18 improves bladder function and alleviates pain by inhibiting TRPV1.
Consistent with the findings of Yang, we found that the expression of TRPV1 was upregulated in the CYP group.63 We found that GPR18 and TRPV1 were coexpressed in small- and medium-sized neurons in the L6-S1 DRGs, providing indirect evidence that GPR18 might function by inhibiting TRPV1. Calcium imaging showed that capsaicin induced a stronger elevation of the intracellular calcium concentration in the CYP group than in the Con group. Consistent with the findings of Perna, we found that the activation of GPR18 with RvD2 inhibited the capsaicin-induced increase in the intracellular calcium concentration and that O-1918 reversed the effect of RvD2 in reducing the intracellular calcium concentration.16
TRPV1 contains many phosphorylation sites and can be phosphorylated in a PKA- or PKC-dependent manner to enhance its activity. For example, histamine enhances TRPV1 activity by activating histamine receptors, leading to increased intracellular PKA and PKC levels.52,64 Gαi-coupled G protein-coupled receptors (GPCRs) are known to transmit inhibitory signals, such as reducing the intracellular calcium concentration. GPR18, a GPCR, is coupled to Gαi.16 Balemans concluded that activation of Gαi-coupled GPCRs by resolvins inhibits adenylyl cyclase expression and subsequently downregulates PKA expression, resulting in the dephosphorylation of TRPV1.65 In our experiment, we hypothesized that RvD2 activates GPR18 to inhibit TRPV1 by inhibiting the cAMP/PKA signalling pathway, but this hypothesis requires further experimental confirmation.
Conclusion
In summary, the present study suggests that activation of GPR18 by RvD2 can alleviate hyperalgesia and improve bladder function in CYP-induced cystitis, possibly through inhibition of TRPV1. Our results suggest that GPR18 might be a potential therapeutic target for IC/BPS.
Acknowledgments
The authors thank Chengfei Yang and Qian Liu for their help with experimental techniques.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No. 81900690) and the Natural Science Foundation of Chongqing (No. cstc2020jcyj-msxmX0065).
Ethics Approval
The animal study was reviewed and approved by Research Council and Animal Care and Use Committee of Army Medical University (approval no. SYXK20070002).
Disclosure
The authors stated that they had no financial or non-financial conflicts of interest with respect to this work.
References
- 1.Hanno PM, Burks DA, Clemens JQ, et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185(6):2162–2170. doi: 10.1016/j.juro.2011.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemens JQ, Link CL, Eggers PW, Kusek JW, Nyberg LM Jr., McKinlay JB. Prevalence of painful bladder symptoms and effect on quality of life in black, Hispanic and white men and women. J Urol. 2007;177(4):1390–1394. doi: 10.1016/j.juro.2006.11.084 [DOI] [PubMed] [Google Scholar]
- 3.Anger JT, Zabihi N, Clemens JQ, Payne CK, Saigal CS, Rodriguez LV. Treatment choice, duration, and cost in patients with interstitial cystitis and painful bladder syndrome. Int Urogynecol J. 2011;22(4):395–400. doi: 10.1007/s00192-010-1252-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riedl C, Engelhardt P, Schwarz B. Treatment costs of bladder pain syndrome/interstitial cystitis in Austria: a pharmacoeconomic approach following current guidelines. Clin Drug Investig. 2013;33(10):737–742. doi: 10.1007/s40261-013-0119-4 [DOI] [PubMed] [Google Scholar]
- 5.Peyronnet B, Mironska E, Chapple C, et al. A comprehensive review of overactive bladder pathophysiology: on the way to tailored treatment. Eur Urol. 2019;75(6):988–1000. doi: 10.1016/j.eururo.2019.02.038 [DOI] [PubMed] [Google Scholar]
- 6.Patnaik SS, Lagana AS, Vitale SG, et al. Etiology, pathophysiology and biomarkers of interstitial cystitis/painful bladder syndrome. Arch Gynecol Obstet. 2017;295(6):1341–1359. doi: 10.1007/s00404-017-4364-2 [DOI] [PubMed] [Google Scholar]
- 7.Akiyama Y, Luo Y, Hanno PM, Maeda D, Homma Y. Interstitial cystitis/bladder pain syndrome: the evolving landscape, animal models and future perspectives. Int J Urol. 2020;27(6):491–503. doi: 10.1111/iju.14229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4(11):873–884. doi: 10.1038/nrn1247 [DOI] [PubMed] [Google Scholar]
- 9.Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain. 2015;16(7):616–627. doi: 10.1016/j.jpain.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14(2):136–148. doi: 10.1016/j.jpain.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corey-Bloom J, Wolfson T, Gamst A, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012;184(10):1143–1150. doi: 10.1503/cmaj.110837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SD, Cho KJ, Kim JC. Expression of cannabinoid 1 and, 2 receptors and the effects of cannabinoid 1 and, 2 receptor agonists on detrusor overactivity associated with bladder outlet obstruction in rats. BMC Urol. 2017;17(1):121. doi: 10.1186/s12894-017-0313-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goncalves dos Santos G, Li R, Ng MPE, et al. CB1 receptor-dependent desensitisation of TRPV1 channels contributes to the analgesic effect of dipyrone in sensitised primary sensory neurons. Br J Pharmacol. 2020;177(20):4615–4626. doi: 10.1111/bph.15170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santha P, Jenes A, Somogyi C, Nagy I. The endogenous cannabinoid anandamide inhibits transient receptor potential vanilloid type 1 receptor-mediated currents in rat cultured primary sensory neurons. Acta Physiol Hung. 2010;97(2):149–158. doi: 10.1556/APhysiol.97.2010.2.1 [DOI] [PubMed] [Google Scholar]
- 15.Ye Y, Scheff NN, Bernabe D, et al. Anti-cancer and analgesic effects of resolvin D2 in oral squamous cell carcinoma. Neuropharmacology. 2018;139:182–193. doi: 10.1016/j.neuropharm.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 16.Perna E, Aguilera-Lizarraga J, Florens MV, et al. Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS. Gut. 2021;70(7):1275–1286. doi: 10.1136/gutjnl-2020-321530 [DOI] [PubMed] [Google Scholar]
- 17.Boucher M, Meen M, Codron JP, Coudore F, Kemeny JL, Eschalier A. Cyclophosphamide-induced cystitis in freely-moving conscious rats: behavioral approach to a new model of visceral pain. J Urol. 2000;164(1):203–208. doi: 10.1097/00005392-200007000-00061 [DOI] [PubMed] [Google Scholar]
- 18.Zhang LY, Liu ZH, Zhu Q, et al. Resolvin D2 relieving radicular pain is associated with regulation of inflammatory mediators, Akt/GSK-3beta signal pathway and GPR18. Neurochem Res. 2018;43(12):2384–2392. doi: 10.1007/s11064-018-2666-9 [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Tonello R, Im ST, et al. Resolvin D3 controls mouse and human TRPV1-positive neurons and preclinical progression of psoriasis. Theranostics. 2020;10(26):12111–12126. doi: 10.7150/thno.52135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanteri-Minet M, Bon K, de Pommery J, Michiels JF, Menetrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-Fos and Krox-24 proteins. Exp Brain Res. 1995;105(2):220–232. doi: 10.1007/BF00240958 [DOI] [PubMed] [Google Scholar]
- 21.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9 [DOI] [PubMed] [Google Scholar]
- 22.Charrua A, Cruz CD, Cruz F, Avelino A. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol. 2007;177(4):1537–1541. doi: 10.1016/j.juro.2006.11.046 [DOI] [PubMed] [Google Scholar]
- 23.Charrua A, Cruz CD, Jansen D, Rozenberg B, Heesakkers J, Cruz F. Co-administration of transient receptor potential vanilloid 4 (TRPV4) and TRPV1 antagonists potentiate the effect of each drug in a rat model of cystitis. BJU Int. 2015;115(3):452–460. doi: 10.1111/bju.12861 [DOI] [PubMed] [Google Scholar]
- 24.Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100(4):685–688. doi: 10.1016/s0306-4522(00)00389-4 [DOI] [PubMed] [Google Scholar]
- 25.Consroe P, Musty R, Rein J, Tillery W, Pertwee R. The perceived effects of smoked cannabis on patients with multiple sclerosis. Eur Neurol. 1997;38(1):44–48. doi: 10.1159/000112901 [DOI] [PubMed] [Google Scholar]
- 26.Merriam FV, Wang ZY, Guerios SD, Bjorling DE. Cannabinoid receptor 2 is increased in acutely and chronically inflamed bladder of rats. Neurosci Lett. 2008;445(1):130–134. doi: 10.1016/j.neulet.2008.08.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakali E, McDonald J, Elliott RA, Lambert DG, Tincello DG. Cannabinoid receptor expression in the bladder is altered in detrusor overactivity. Int Urogynecol J. 2016;27(1):129–139. doi: 10.1007/s00192-015-2802-x [DOI] [PubMed] [Google Scholar]
- 28.Homma Y. Interstitial cystitis, bladder pain syndrome, hypersensitive bladder, and interstitial cystitis/bladder pain syndrome - clarification of definitions and relationships. Int J Urol. 2019;26(Suppl 1):20–24. doi: 10.1111/iju.13970 [DOI] [PubMed] [Google Scholar]
- 29.Farquhar-Smith WP, Rice AS. Administration of endocannabinoids prevents a referred hyperalgesia associated with inflammation of the urinary bladder. Anesthesiology. 2001;94(3):507–13; discussion 6A. doi: 10.1097/00000542-200103000-00023 [DOI] [PubMed] [Google Scholar]
- 30.Wang ZY, Wang P, Bjorling DE. Activation of cannabinoid receptor 2 inhibits experimental cystitis. Am J Physiol Regul Integr Comp Physiol. 2013;304(10):R846–R853. doi: 10.1152/ajpregu.00585.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farquhar-Smith WP, Jaggar SI, Rice AS. Attenuation of nerve growth factor-induced visceral hyperalgesia via cannabinoid CB(1) and CB(2)-like receptors. Pain. 2002;97(1–2):11–21. doi: 10.1016/s0304-3959(01)00419-5 [DOI] [PubMed] [Google Scholar]
- 32.Gu X, Mei F, Liu Y, Zhang R, Zhang J, Ma Z. Intrathecal administration of the cannabinoid 2 receptor agonist JWH015 can attenuate cancer pain and decrease mRNA expression of the 2B subunit of N-methyl-D-aspartic acid. Anesth Analg. 2011;113(2):405–411. doi: 10.1213/ANE.0b013e31821d1062 [DOI] [PubMed] [Google Scholar]
- 33.Cui JH, Kim WM, Lee HG, Kim YO, Kim CM, Yoon MH. Antinociceptive effect of intrathecal cannabinoid receptor agonist WIN 55,212-2 in a rat bone tumor pain model. Neurosci Lett. 2011;493(3):67–71. doi: 10.1016/j.neulet.2010.12.052 [DOI] [PubMed] [Google Scholar]
- 34.Fabisiak A, Fabisiak N, Mokrowiecka A, et al. Novel selective agonist of GPR18, PSB-KK-1415 exerts potent anti-inflammatory and anti-nociceptive activities in animal models of intestinal inflammation and inflammatory pain. Neurogastroenterol Motil. 2021;33(3):e14003. doi: 10.1111/nmo.14003 [DOI] [PubMed] [Google Scholar]
- 35.Huang SM, Bisogno T, Petros TJ, et al. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J Biol Chem. 2001;276(46):42639–42644. doi: 10.1074/jbc.M107351200 [DOI] [PubMed] [Google Scholar]
- 36.Wrobel A, Zapala L, Zapala P, Piecha T, Radziszewski P. The effect of O-1602, a GPR55 agonist, on the cyclophosphamide-induced rat hemorrhagic cystitis. Eur J Pharmacol. 2020;882:173321. doi: 10.1016/j.ejphar.2020.173321 [DOI] [PubMed] [Google Scholar]
- 37.Wrobel A, Szopa A, Serefko A, Poleszak E, Novel A. Alternative in the treatment of detrusor overactivity? In vivo activity of O-1602, the newly synthesized agonist of GPR55 and GPR18 Cannabinoid receptors. Molecules. 2020;25(6):1384. doi: 10.3390/molecules25061384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wrobel A, Serefko A, Szopa A, Ulrich D, Poleszak E, Rechberger T. O-1602, an agonist of atypical Cannabinoid receptors GPR55, reverses the symptoms of depression and detrusor overactivity in rats subjected to corticosterone treatment. Front Pharmacol. 2020;11:1002. doi: 10.3389/fphar.2020.01002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466. doi: 10.1038/nrn2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology. 2002;59(5 Suppl 1):61–67. doi: 10.1016/s0090-4295(01)01639-9 [DOI] [PubMed] [Google Scholar]
- 41.Nazif O, Teichman J, Gebhart G. Neural upregulation in interstitial cystitis. Urology. 2007;69(4):24–33. doi: 10.1016/j.urology.2006.08.1108 [DOI] [PubMed] [Google Scholar]
- 42.Keast JR, De Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319(4):615–623. doi: 10.1002/cne.903190411 [DOI] [PubMed] [Google Scholar]
- 43.Merrill L, Gonzalez EJ, Girard BM, Vizzard MA. Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat Rev Urol. 2016;13(4):193–204. doi: 10.1038/nrurol.2016.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu BL, Yang F, Zhan HL, et al. Increased severity of inflammation correlates with elevated expression of TRPV1 nerve fibers and nerve growth factor on interstitial cystitis/bladder pain syndrome. Urol Int. 2014;92(2):202–208. doi: 10.1159/000355175 [DOI] [PubMed] [Google Scholar]
- 45.Dickson A, Avelino A, Cruz F, Ribeiro-da-silva A. Peptidergic sensory and parasympathetic fiber sprouting in the mucosa of the rat urinary bladder in a chronic model of cyclophosphamide-induced cystitis. Neuroscience. 2006;141(3):1633–1647. doi: 10.1016/j.neuroscience.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 46.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19(11):4644–4653. doi: 10.1523/jneurosci.19-11-04644.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y, de Groat WC. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol. 2008;294(5):F1146–56. doi: 10.1152/ajprenal.00592.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425(1):545–562. doi: 10.1113/jphysiol.1990.sp018117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeda M, Mochizuki T, Yoshiyama M, et al. Sensor mechanism and afferent signal transduction of the urinary bladder: special focus on transient receptor potential ion channels. Low Urin Tract Symptoms. 2010;2(2):51–60. doi: 10.1111/j.1757-5672.2010.00074.x [DOI] [PubMed] [Google Scholar]
- 50.Dang K, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced cystitis reduces ASIC channel but enhances TRPV1 receptor function in rat bladder sensory neurons. J Neurophysiol. 2013;110(2):408–417. doi: 10.1152/jn.00945.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coelho A, Wolf-Johnston AS, Shinde S, et al. Urinary bladder inflammation induces changes in urothelial nerve growth factor and TRPV1 channels. Br J Pharmacol. 2015;172(7):1691–1699. doi: 10.1111/bph.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grundy L, Caldwell A, Garcia Caraballo S, et al. Histamine induces peripheral and central hypersensitivity to bladder distension via the histamine H1 receptor and TRPV1. Am J Physiol Renal Physiol. 2020;318(2):F298–F314. doi: 10.1152/ajprenal.00435.2019 [DOI] [PubMed] [Google Scholar]
- 53.Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005;65(2):400–405. doi: 10.1016/j.urology.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 54.Brady CM, Apostolidis AN, Harper M, et al. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004;93(6):770–776. doi: 10.1111/j.1464-410X.2003.04722.x [DOI] [PubMed] [Google Scholar]
- 55.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain. 2008;139(1):158–167. doi: 10.1016/j.pain.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 56.Dornelles FN, Andrade EL, Campos MM, Calixto JB. Role of CXCR2 and TRPV1 in functional, inflammatory and behavioural changes in the rat model of cyclophosphamide-induced haemorrhagic cystitis. Br J Pharmacol. 2014;171(2):452–467. doi: 10.1111/bph.12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walczak JS, Price TJ, Cervero F. Cannabinoid CB1 receptors are expressed in the mouse urinary bladder and their activation modulates afferent bladder activity. Neuroscience. 2009;159(3):1154–1163. doi: 10.1016/j.neuroscience.2009.01.050 [DOI] [PubMed] [Google Scholar]
- 58.Walczak JS, Cervero F. Local activation of cannabinoid CB(1) receptors in the urinary bladder reduces the inflammation-induced sensitization of bladder afferents. Mol Pain. 2011;7:31. doi: 10.1186/1744-8069-7-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christie S, Zagorodnyuk V. CB2 cannabinoid receptor agonist selectively inhibits the mechanosensitivity of mucosal afferents in the Guinea pig bladder. Am J Physiol Renal Physiol. 2021;320(5):F859–F865. doi: 10.1152/ajprenal.00065.2021 [DOI] [PubMed] [Google Scholar]
- 60.Aizawa N, Gandaglia G, Hedlund P, et al. URB937, a peripherally restricted inhibitor for fatty acid amide hydrolase, reduces prostaglandin E2 -induced bladder overactivity and hyperactivity of bladder mechano-afferent nerve fibres in rats. BJU Int. 2016;117(5):821–828. doi: 10.1111/bju.13223 [DOI] [PubMed] [Google Scholar]
- 61.Gratzke C, Streng T, Park A, et al. Distribution and function of cannabinoid receptors 1 and 2 in the rat, monkey and human bladder. J Urol. 2009;181(4):1939–1948. doi: 10.1016/j.juro.2008.11.079 [DOI] [PubMed] [Google Scholar]
- 62.Patel HJ, Birrell MA, Crispino N, et al. Inhibition of Guinea-pig and human sensory nerve activity and the cough reflex in Guinea-pigs by cannabinoid (CB2) receptor activation. Br J Pharmacol. 2003;140(2):261–268. doi: 10.1038/sj.bjp.0705435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y, Zhang H, Lu Q, et al. Suppression of adenosine A2a receptors alleviates bladder overactivity and hyperalgesia in cyclophosphamide-induced cystitis by inhibiting TRPV1. Biochem Pharmacol. 2021;183:114340. doi: 10.1016/j.bcp.2020.114340 [DOI] [PubMed] [Google Scholar]
- 64.Wouters MM, Balemans D, Van Wanrooy S, et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology. 2016;150(4):875–87 e9. doi: 10.1053/j.gastro.2015.12.034 [DOI] [PubMed] [Google Scholar]
- 65.Balemans D, Boeckxstaens G, Talavera K, Wouters M. Transient receptor potential ion channel function in sensory transduction and cellular signaling cascades underlying visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2017;312(6):G635–G648. doi: 10.1152/ajpgi.00401.2016 [DOI] [PubMed] [Google Scholar]