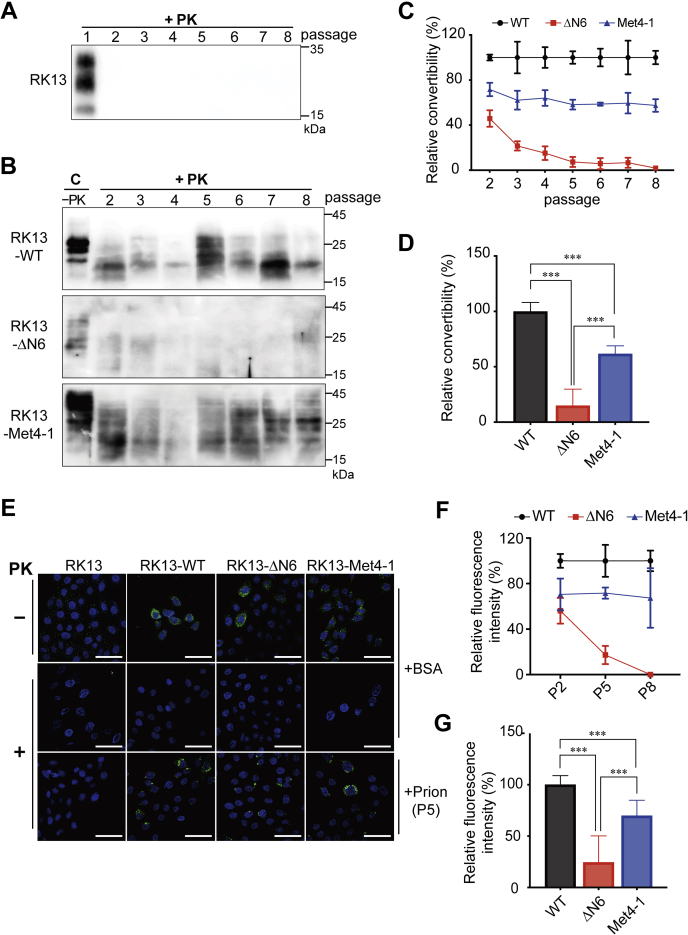
Figure 4.
Prion infection in RK13-WT, RK13-Met4-1, and RK13-ΔN6 cells. RK13 cells expressing WT-PrP, Met4-1-PrP, or ΔN6-PrP were incubated with 0.5% brain homogenate prepared from a mouse suffering from terminal prion disease and then subjected to seven passages. The initial prion-infected culture was designated as passage 1 (P1). PK-resistant PrPSc in untransfected RK13 cells (A) and in WT-PrP, Met4-1-PrP, or ΔN6-PrP cells (B) were determined by PK digestion and Western blotting with the 3F10 anti-PrP antibody. C represents uninfected control cell lysates. Densitometric analysis of the PK-resistant PrP were shown in (C) and (D) (n = 3 independent prion infections of each stable RK13 cell line). Relative PrP convertibility = PK resistant PrP/PrP without PK digestion. The PrP conversion rate of RK13-WT cells was set as 100%. E, passage 5 (P5) cells were analyzed by immunofluorescence staining with the 3F10 anti-PrP antibody with and without PK digestion as indicated. Negative control cells were incubated with 0.5% BSA and analyzed in the same manner. The scale bar represents 100 μm. F, densitometric analysis of passages 2, 5, and 8 (P2, P5, and P8) cells that were immunofluorescently stained with the 3F10 anti-PrP antibody (n = 3 independent prion infections of each stable RK13 cell line). The relative fluorescence intensity of PK-resistant PrP in RK13-WT cells was set as 100%. G, comparison of mean relative fluorescence intensity of RK13-WT, RK13-Met4-1, and RK13-ΔN6 (n = 3). Statistical significance was determined by one-way ANOVA followed by Tukey's multiple comparison test. ∗∗∗ represents p < 0.01. Error bars indicate standard deviations. Results in C and F were from three independent prion infection experiments. PK, proteinase K; PrP, prion protein.