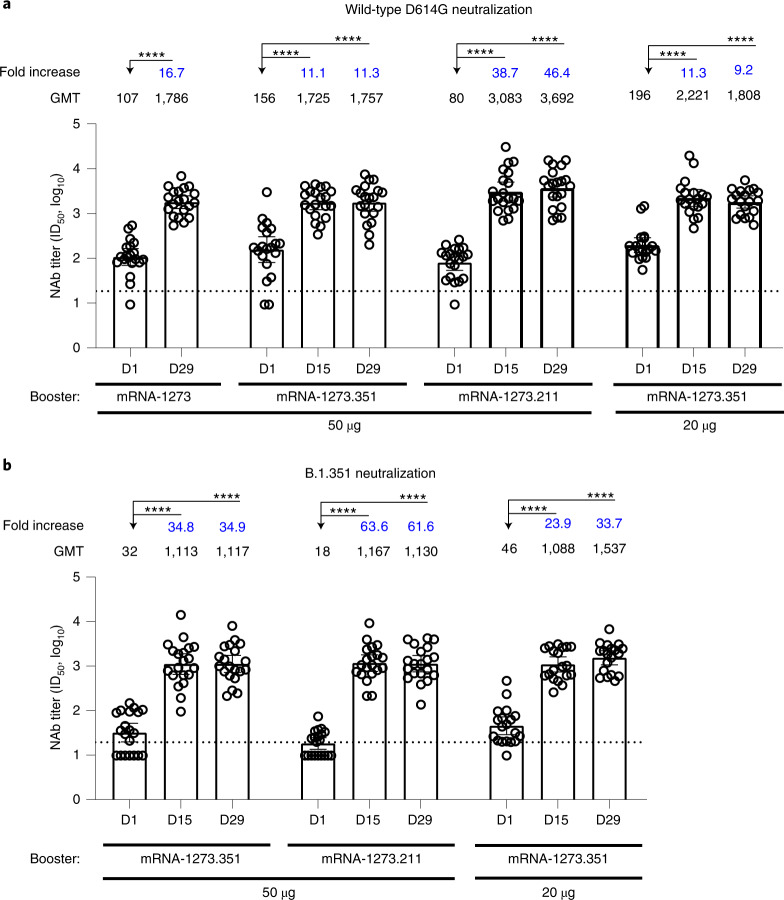
Fig. 3. Neutralization of wild-type D614G and B.1.351 by participant serum collected immediately before and after boosters, as measured by the lentiviral-based PsVN assay.
Wild-type D614G neutralization (a) and B.1.351 neutralization (b) in a validated recombinant lentivirus-based SARS-CoV-2 pseudovirus assay by serum from participants (n = 20 participants per booster cohort). Sera samples were collected immediately before receiving a booster (day 1) and on days 15 and 29 after the booster dose of mRNA-1273 (50 µg), mRNA-1273.351 (50 or 20 µg) or mRNA-1273.211 (50 µg). Participant sera in the mRNA-1273 (50 µg) booster group were not assessed in the B.1.351 assay. Data are presented as the geometric mean neutralizing antibody titers with 95% confidence intervals. The titers for individual participants are indicated with circles. The fold increases in titers measured at days 15 and 29 versus titers measured before the booster dose are shown. The horizonal dotted lines indicate the LLOQ. Generalized linear model was used to compare neutralization titers among groups; log10 titer was regressed on group, and an individual-specific random effect was included to account for individual specific variability. Two-sided t-test was used for post hoc group comparisons. Sidak’s method was used to adjust the P values for multiple comparisons. Statistical significance was determined at α < 0.01. ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05. NAb, neutralizing antibody.