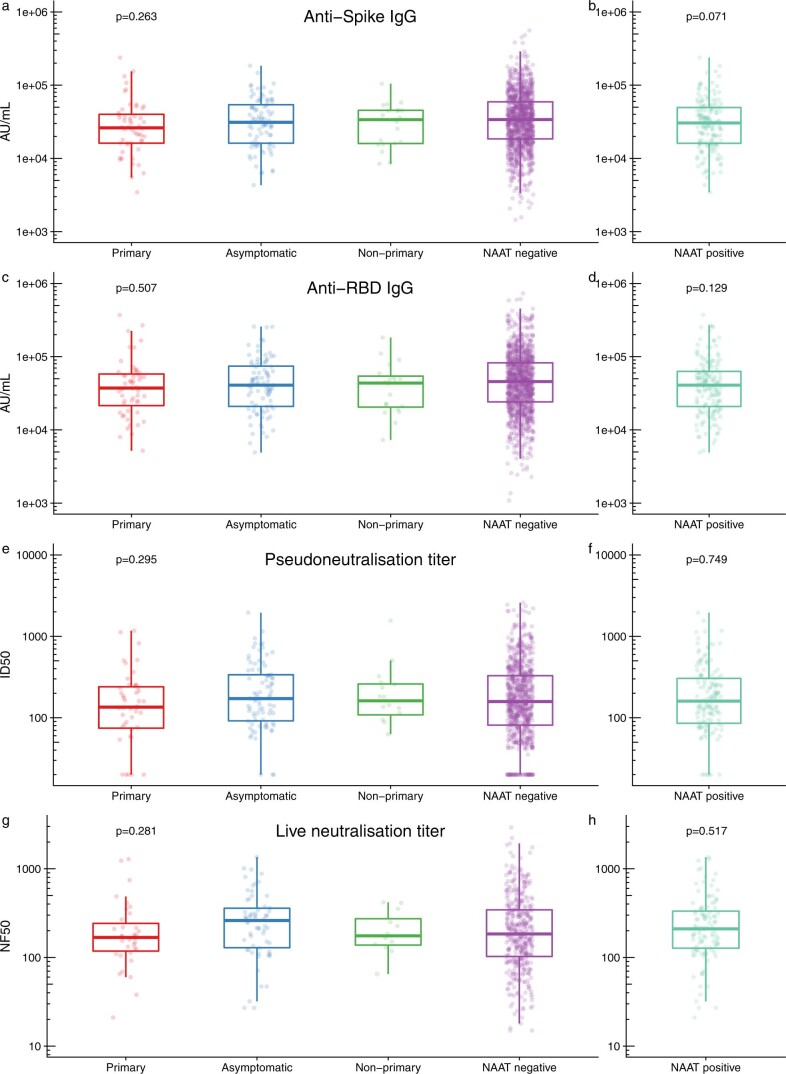
Extended Data Fig. 2. Immune markers measured at day 28 post-second dose, in primary symptomatic, asymptomatic, non-primary cases, NAAT positive cases and NAAT negative non-cases.
a: N = 1155 NAAT negative, 52 primary, 91 asymptomatic and 20 non-primary, b: N = 163 NAAT positive participants’ anti-spike IgG measured at 28 days post boost; c: N = 1155 negative, 52 primary, 91 asymptomatic and 20 non-primary, d: N = 163 NAAT positive participants’ anti-RBD IgG measured at 28 days post boost; e: N = 828 NAAT negative, 47 primary, 86 asymptomatic and 16 non-primary, f: N = 149 NAAT positive participants’ pseudovirus neutralisation titre measured at 28 days post boost; g: N = 412 negative, 36 primary, 62 asymptomatic and 12 non-primary, h: N = 110 NAAT positive participants’ live neutralisation titre measured at 28 days post boost. a–h: minima: smallest value; maxima: largest value; centre: median value; bounds of box: 25% and 75% quartile value; upper/lower whisker extends from the hinge to the largest/smallest value no further than 1.5 * inter-quartile range from the hinge. IgG: Immunoglobulin G; RBD: receptor binding domain. Primary symptomatic cases: NAAT+ with at least one COVID symptom (cough, fever, shortness of breath, anosmia, aguesia). Asymptomatic cases: NAAT+ on weekly self-swab with no symptoms recorded. Non-primary cases: NAAT+ with only non-primary COVID symptoms (for example nausea, diarrhoea). P-value estimated by one-way ANOVA test comparing between primary, asymptomatic, non-primary cases and NAAT negative non-cases and by two sample t-test comparing between NAAT positive cases and NAAT negative non-cases (two-sided).