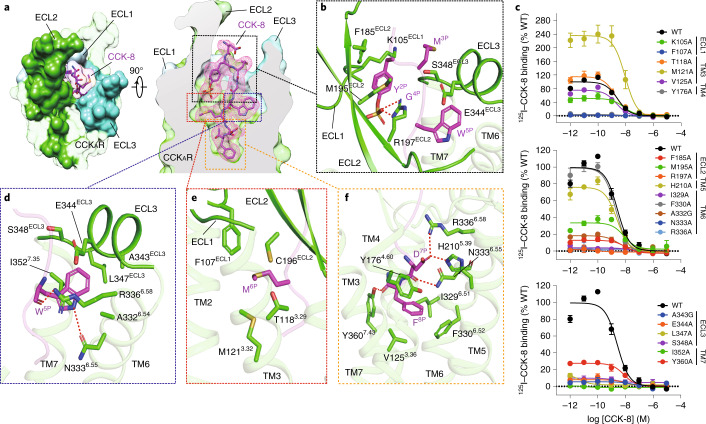
Fig. 2. Recognition of sulfated CCK-8 by CCKAR.
a, CCK-8 sits in the orthosteric binding pocket of CCKAR, as shown in an extracellular view (left) and side view (right). The density map of CCK-8 is shown as a magenta mesh, and CCK-8 is displayed as magenta sticks. CCKAR is shown in green as a cutaway surface (right). ECL1 (light blue), ECL2 (lime green) and ECL3 (turquoise) are highlighted as solid surfaces. b, Detailed interactions between sulfated CCK-8 and three extracellular loops of CCKAR. c, Effects of mutations in the receptor ligand-binding pocket on CCK-8 binding activity assessed by a radiolabeled ligand-binding assay. Data are presented as mean ± s.e.m. of three independent experiments (n = 3), except for the WT (n = 4), and conducted in triplicate. Competition curves of mutants from ECL1, TM3 and TM4 (upper), ECL2, TM5 and TM6 (middle), and ECL3 and TM7 (bottom) compared to WT CCKAR are shown. d, Recognition of CCK-8 by the deep hydrophobic cavity beneath ECL3 of CCKAR. e, Recognition of CCK-8 by the shallow hydrophobic cavity beneath ECL1 and ECL2 of CCKAR. f, Recognition of CCK-8 by the bottom TMD region of CCKAR. Key interaction residues from CCKAR are shown as green sticks, and the receptor is shown in cartoon presentation. Polar interactions are indicated as red dashed lines.