Abstract
White wine is among the most widely consumed alcoholic beverages. Varietal discrimination of wines has received increasing attention. Today's consumers require a sense of authenticity and are deterred by falsehood or misrepresentation in product marketing. However, wine can involve various types of frauds, which directly affects the distribution of wine in national and international markets. Right-angle fluorescence spectroscopy is a simple and rapid analytical technique that in combination with chemometric algorithms, constitutes a novel method for wine authentication. In this study, the stepwise-Linear Discriminant Analysis algorithm was applied in three representative spectral regions related to phenolic compounds for the purpose of distinguishing white wines according to the grape variety. The wavelength at 310 nm attributed to the hydroxycinnamic acids and stilbene provided a higher classification rate (95.5%) than the λex 280 and 295 nm regions (79.8%), suggesting that these compounds are highly related to the botanical origin of samples. The chemometric models were validated utilizing cross-validation and an external validation set to enhance the robustness of the proposed methodology. The above-mentioned methodology constitutes a powerful tool for the varietal discrimination of white wines and can be used in industrial setting. The ultimate goal of this study is to contribute to the efforts towards the authentication of Greek white wine which will eventually support producers and suppliers to remain competitive and simultaneously protect the consumers from fraudulent practices.
Keywords: White wines, Right-angle fluorescence spectroscopy, Phenolic compounds, Stepwise linear discriminant analysis, Authenticity
Graphical abstract
Highlights
-
•
Acquisition of fluorescence fingerprint from 140 commercial Greek white wines.
-
•
Qualitative study of the main fluorometric regions by standard phenolic compounds.
-
•
Distinguish autochthonous commercial Greek white wines based on EEMs using SLDA.
-
•
Development of a novel, cheap, and rapid methodology for wine authentication.
-
•
Reliable chemometric model by internal and external validation.
1. Introduction
The COVID-19 crisis has had a significant impact on global wine sales, leading to a drop in sales of almost 10%, with a 1.1% volume decline around the world which has not been fully compensated for by e-commerce sales (Platform and Ciberobn, 2020). Nevertheless, the wine market's global revenue is projected to reach a forecasted 9.2% Compound Annual Growth Rate (CAGR) in 2025 (Wine market revenue worldwide 2012-2025 | Statista,). Greece ranks seventh in the EU in terms of production volume, responsible for approximately 2% of global production, and Greek wine market revenue is projected to reach 4.6% CAGR in 2025 (Wine market revenue worldwide 2012-2025 | Statista,). The wine industry in Greece includes a few large wineries, many medium-sized and small local wineries, and associations of agricultural cooperatives. Despite the long tradition of winemaking and the many well-known Protected Designation of Origin (PDO) wines, Greece, unlike other southern European Union (EU) countries has not yet fully exploited the industry's export potential. Major problems that hinder the achievement of the above outcome are the various types of fraud and numerous instances of falsified labels present in the wine market (Kamiloglu, 2019).
Two widely used approaches for determining the authenticity of wine are chromatographic and spectroscopic techniques (Anjos et al., 2020; Basalekou et al., 2020; Magdas et al., 2019a, Magdas et al., 2019b; Popîrdă et al., 2021; Ranaweera et al., 2021a). Chromatographic techniques such as gas chromatography-mass spectrometry (GC-MS) (Tourtoglou et al., 2014; Tufariello et al., 2019; Ziółkowska et al., 2016) and liquid chromatography-mass spectrometry (LC-MS) (Medel-Marabolí et al., 2021; Tzachristas et al., 2021; Valentin et al., 2020) allow the simultaneous determination of several compounds in one run, with high accuracy and reproducibility. However, a disadvantage of these techniques is that they require sample extraction procedures, which are often regarded as time-consuming and sometimes include toxic solvents. In addition, these techniques require expensive and complex systems as well as skilled operators to perform tests and interpret results.
Spectroscopic techniques provide a reliable solution due to their rapid, non-destructive, and non-invasive preparation of samples and the fact that they do not alter the environmental fingerprint. The spectroscopic techniques that have been utilized for botanical and geographical wine differentiation include ultraviolet–visible spectroscopy (UV–Vis) (Azcarate et al., 2013), nuclear magnetic resonance (NMR) (Mascellani et al., 2021), Raman (Magdas et al., 2019a, Magdas et al., 2019b), near-infrared (NIR), and mid-infrared (MIR) (Basalekou et al., 2017; Hu et al., 2019).
Fluorescence spectroscopy is simple and is 100–1000 times more sensitive than other spectroscopic techniques (Strasburg and Ludescher, 1995); as a result it has been deemed a useful tool in botanical (Azcarate et al., 2015; Ranaweera et al., 2021b; Sádecká and Jakubíková, 2020; Suciu et al., 2019) or geographical (Airado-Rodŕiguez et al., 2009; Dufour et al., 2006; Ranaweera et al., 2021c) wine authentication. This tool becomes even more powerful and promising when the spectrum data is combined with chemometric methods such as linear discriminant analysis (LDA), principal component analysis (PCA) (Chen et al., 2021; Sádecká and Jakubíková, 2020), soft independent modeling of class analogy (SIMCA), parallel factor analysis (PARAFAC) (Suciu et al., 2019), support vector machine discriminant analysis (SVMDA), or extreme gradient boosting discriminant analysis (XGBDA) (Ranaweera et al., 2021c). Research reports on the application of chemometric methods in combination with fluorescence spectroscopy are limited and therefore more efforts need to be made in this direction which will eventually reinforce the wine authenticity establishment in international markets (Ranaweera et al., 2021a).
Several monovarietal white wines are made from indigenous varieties of PDO or in protected geographical indication (PGI) regions. White wines derived from Malagouzia, Moschofilero, Asyrtiko, Vidiano, and Savatiano constitute the most commercially successful Greek white wines and are highly appreciated by consumers. Despite the popularity of these wines, no scientific articles have yet been published which investigate the use of fluorescence spectrometry to authenticate Greek wine products.
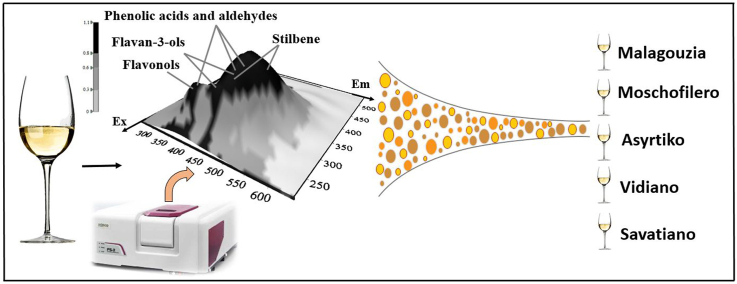
The aim of this work was to investigate possible chemometric models based on fluorescence excitation-emission matrix (EEM) spectra information on the intrinsic fluorophore compounds of five autochthonous Greek wines. The novelty behind this approach lies in the development of a simple and rapid methodology based on right-angle geometry fluorescence spectroscopy and the stepwise-LDA algorithm that can be used for routine analysis in the industry, thereby enhancing the export potential of the most commercially successful Greek white wines.
2. Materials and methods
2.1. White wine samples
A total of 140 monovarietal white wines were derived from five autochthonous botanical origins (20 Malagouzia, 35 Moschofilero, 14 Asyrtiko, 22 Vidiano, and 49 Savatiano). All samples were emanated from vine-growing areas of Greek mainland. However, there are no geographically isolated areas that could affect the results. Samples were purchased directly from producers during the 2020 vinification year and were kept in the dark at 4 °C in hermetically closed glass bottles until further analysis. The recording of EEMs spectra were carried out after three weeks.
2.2. Reagents and solutions
To achieve the attribution of the fluorescence spectral regions, phenolic compounds standards (98–99% purities), including methyl syringate, caffeic, ferulic, syringic, and vanillic acid were purchased from Sigma-Aldrich (Steinheim, Germany). Also, kaempferol, catechin, and phenolic acids (sinapic, gentisic, ellagic, chlorogenic, homogentisic, p-coumaric, protocatechuic, gallic) were purchased from Extrasynthese (Geney, France). Standards were dissolved in MS-grade acetonitrile solution (0.01 mg/mL) (Sigma- Aldrch Merck KGaA, Darmstadt, Germany).
2.3. Fluorescence spectroscopy
The analyses were carried out in a FluoroMate FS-2 spectrometer (CE Mark. Scinco, Nieuwegein, NLD) supported by FluoroMasterPlus software (CE Mark. Scinco, Nieuwegein, NLD). The spectrometer was equipped with a continuous wave xenon-arc lamp light source with 500W of output power. The type of electronic transition was S1 → S0 with timescale of 10−9 to 10−6 s. Three-dimensional-emission excitation matrices (3D-EEMs) were recorded in duplicate using a quartz cuvette (10 mm, 3.5 mL) and a right-angle sample holder. Each spectrum acquisition was adjusted the excitation wavelength (λex) from 260 to 520 nm and emission wavelength (λem) from 280 to 620 nm. The slits and intervals of excitation and emission monochromators were set at 5 nm, respectively.
3D-EEM spectra pre-treatment was handled using XLSTAT-3DPlot ver 2019.2.2 (Addinsoft Inc., New York, NY, USA) software. Prior to statistical analysis, each 3D-EEM spectrum was saved as a CSV file, and representative 2D-spectra were selected according to λem max for further treatment. Then, using the “Statistical Spectra” function, the average of the two spectra for each sampled was calculated, and each average spectrum was normalized (unit vector normalization) using the Unscrambler X ver.10.4 (CAMO software AS., Oslo, Norway) software.
2.4. Statistical analysis
A stepwise-LDA algorithm was applied to classify the 89 white wine samples (17 Malagouzia, 18 Moschofilero, 12 Asyrtiko, 12 Vidiano, and 30 Savatiano). Also, in order to assess the ability of the calibration model the rest of 51 samples (3 Malagouzia, 17 Moschofilero, 2 Asyrtiko, 10 Vidiano, and 19 Savatiano) were used for the test set. All samples were randomly allocated into the above sets and all chemometric models were examined using cross-validation and external validation. The statistical analyses were performed using the SPSS v.25 (IBM, SPSS, Statistics) software.
3. Results and discussion
3.1. Fluorescence spectra of standards phenolic compounds and white wines
The EEMs of standard phenolic compounds indicate characteristic spectral regions with λex maxima between 260 and 360 nm. In more detail, at 260–315/315–345 nm (λex/λem) methyl syringate, catechin, and gallic, protocatechuic, syringic, vanillic, homogentisic acid have been identified. In addition, the presence of kaempferol, and caffeic, chlorogenic, p-coumaric, ferulic, sinapic acid at 300–360/380–450 nm (λex/λem) was observed, while some phenolics like ellagic and gentisic acids fluoresce in a wider range at 280–380/400–480 nm (λex/λem). The wavelengths of λex and λem of standard phenolic compounds are presented in Table 1. As the literature reports, λex at 280 nm has been related to catechin, epicatechin, and syringic, gallic, and protocatechuic acids (Rodríguez-Delgado et al., 2001). The spectral region with λex at 325 nm has mainly been linked to hydroxycinnamic acids such as caffeic, caftaric, ferulic, sinapic, and p-coumaric acid (Martin et al., 2017).
Table 1.
The wavelengths of λex and λem of standards phenolic compounds.
| Phenolic compound | λex (nm) | λem (nm) |
|---|---|---|
| Caffeic acid | 310–360 | 410 |
| Chlorogenic acid | 300–360 | 416 |
| p-Coumaric acid | 320–340 | 380 |
| Ferulic acid | 310–360 | 400 |
| Sinapic acid | 300–360 | 415 |
| Ellagic acid | 280–380 | 400 |
| Homogentisic acid | 280–320 | 335 |
| Gallic acid | 265–315 | 345 |
| Protocatechuic acid | 265–315 | 335 |
| Syringic acid | 250–315 | 335 |
| Vanillic acid | 260–315 | 330 |
| Methyl syringate | 250–320 | 340 |
| Gentisic acid | 290–360 | 400 and 475 |
| Kaempferol | 280–320 | 430 and 500 |
| Catechin | 250–310 | 315 |
Wine consists of a complex matrix with several fluorescent compounds corresponding in spectral regions of interest. Phenolic compounds represent well-known fluorescent molecules that are directly related to grape cultivars (Ríos-Reina et al., 2017; Tzachristas et al., 2021). EEM spectra of white wine samples consisted of overlapped regions and therefore decomposing was necessary to be applied. The decomposed ΕΕΜs revealed three PARAFAC components with exhibited maxima in the region of λex 260, 280 and, 320–330 nm (Airado-Rodríguez et al., 2011; Schueuermann et al., 2018).
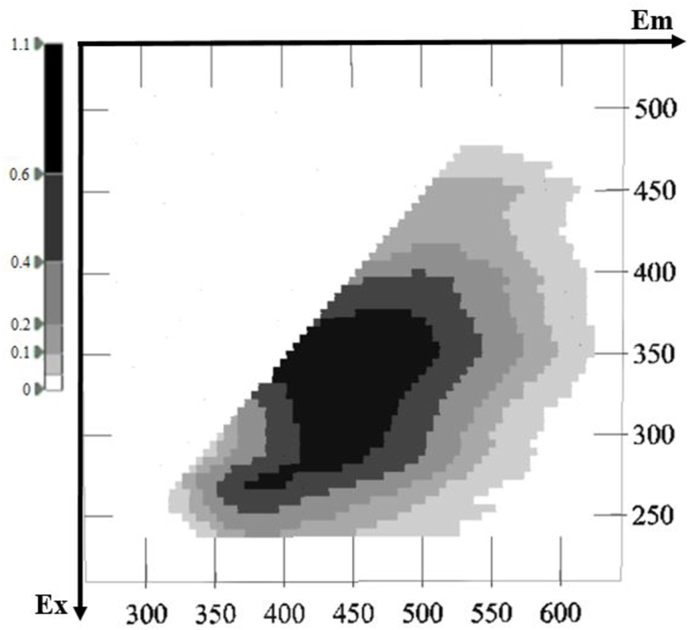
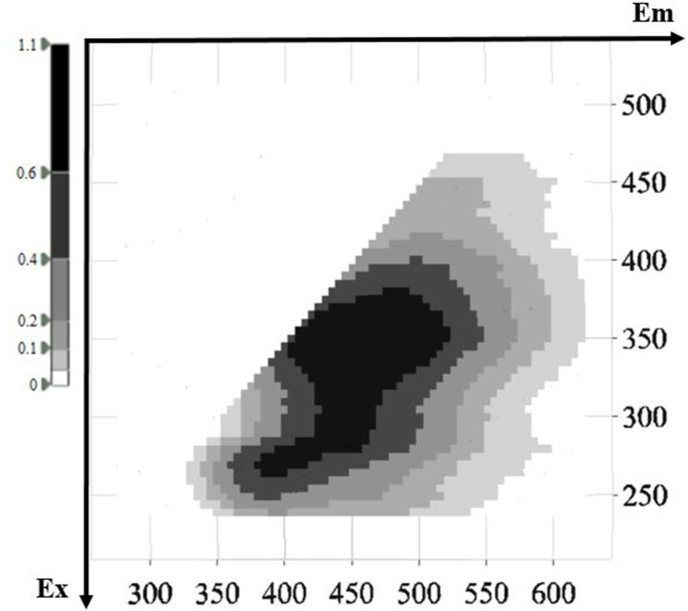
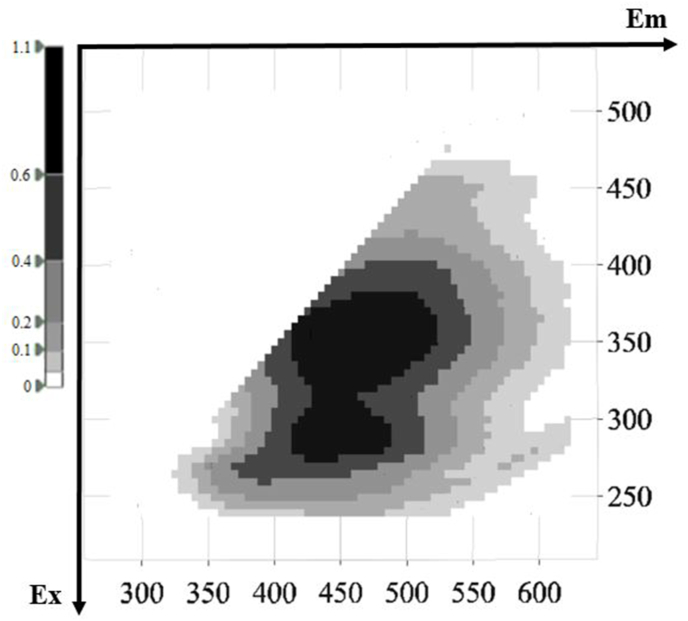
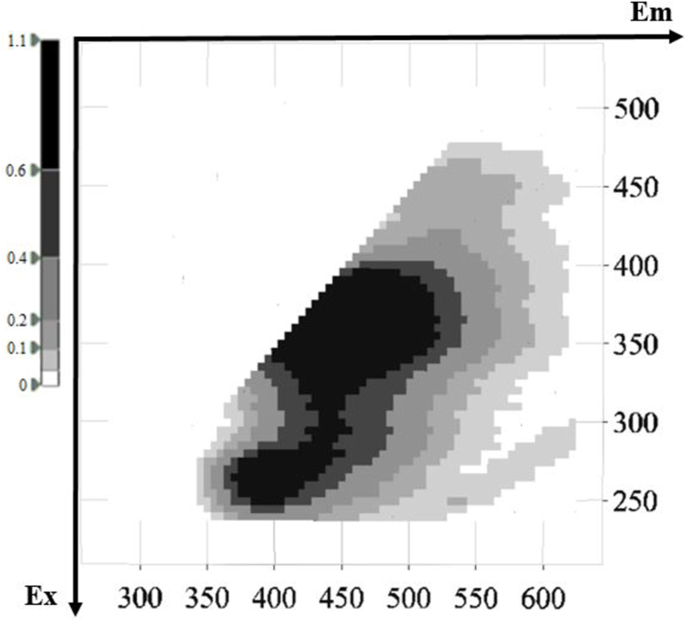
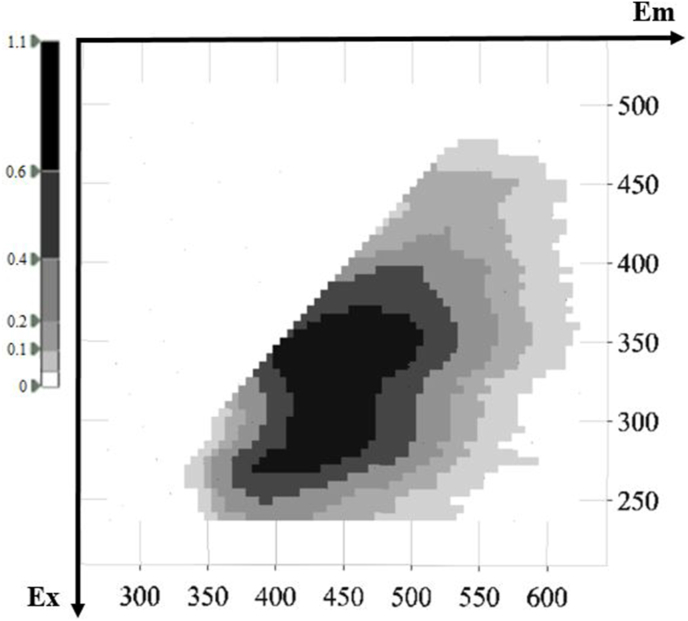
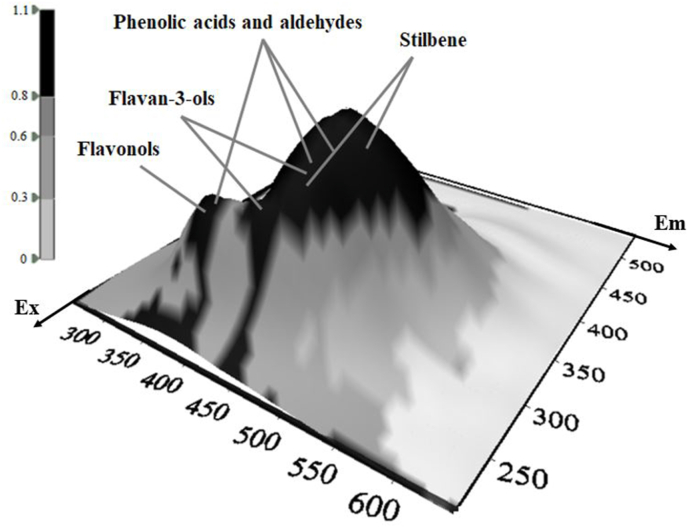
Representative 3D fluorescence spectra from each indigenous Greek variety are presented in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5. As depicted, the fluorescence surfaces are quite similar (Fig. 6). Specifically, the fluorescent region that exhibited excitation with λex ranging between 260 and 320 nm was found to mainly contain non-flavonoids such as cinnamic-like and benzoic-like phenolic acids. However, phenolic aldehydes, flavonols, and flavon-3-ols also appear in this spectral region (Airado-Rodríguez et al., 2011). In more detail, flavonols appeared at λex 260–280 nm with λem 370–420 nm; monomeric and polymeric flavan-3-ols appeared at λex 280–300 nm with λem 310–360 nm; and phenolic acids and aldehydes appeared at λex 260–320 nm and λem 320–440 nm (Airado-Rodríguez et al., 2011).
Fig. 1.
Characteristic fluorescence 3D-EEM spectra of Malagouzia.
Fig. 2.
Characteristic fluorescence 3D-EEM spectra of Moschofilero.
Fig. 3.
Characteristic fluorescence 3D-EEM spectra of Asyrtiko.
Fig. 4.
Characteristic fluorescence 3D-EEM spectra of Vidiano.
Fig. 5.
Characteristic fluorescence 3D-EEM spectra of Savatiano.
Fig. 6.
Representative 3D-EEM spectra of intrinsic fluorochrome compounds in white wine of Savatiano.
These variations that appear in the specific λex/λem pairs are due to the interaction of the different vicinities of compounds and can contribute to the varietal discrimination of white wine samples. Three characteristic spectra at λex 280, 295, and 310 nm were examined in order to develop an alternative novel method to differentiate five varieties of commercial Greek white wine.
3.2. Stepwise LDA based on white wines fluorescence spectra
In order to build chemometric models, the samples (n = 140) were partitioned randomly into two groups. The first group (n = 89) was used to test the ability of a “calibration model” to classify white wine samples according to the grape variety. In addition, this group underwent a cross-validation procedure called an “internal validation”. Following this step, it was essential to control the robustness of the model by testing a test set in an “external validation” step. For this purpose, the second group (n = 51) was used as an independent set to evaluate the discriminative power of the developed models. Each chemometric model was carried out using a stepwise-LDA algorithm based on the Mahalanobis distance. In this way, redundant variables were removed and the variables which were most significant for the development of discrimination functions were identified. In addition, statistical tests such as Wilks Lambda (Λ), eigenvalues, and canonical correlation confirmed the existence of significant differences between the means vectors of the calibration model.
The chemometric models, based on λex = 280 nm and λex = 310 nm demonstrated that eight stepwise steps (p < 0.05) were formed, while seven stepwise steps (p < 0.05) were formed for the chemometric model based on λex = 295 nm. The Eigenvalues, (Λ), and canonical correlations of the discriminant functions that were used in the evaluation of the chemometric models are reported in Supplementary Table 1.
The discriminant percentage of the above-mentioned models at (λex) 280 and 295 nm exhibited quite similar scores (Supplementary Tables 2–3). In particular, calibration, internal, and external validation accuracy rate values were 79.8; 74.2; and 74.5% and 79.8; 73.0; and 76.5% at (λex) 280 and 295 nm, respectively. These wavelength widths covered a range of phenolic components. . High levels of accuracy in the discrimination between the white wine varieties correlated mostly with the presence of hydroxybenzoic acids such as syringic, gallic, vanillic, and protocatechuic acid and to a lesser degree with flavonols and flavan-3-ols such as kaempferol and catechin. Additionally, a smaller level of discriminatory accuracy was due to the presence of gentisic acid (Airado-Rodríguez et al., 2011).
From the application of the stepwise algorithm, the standardized canonical discriminant function coefficients which contributed to distinguishing the white wines in the chemometric models based on fluorescence spectra at λex 280 and 295 nm were found to be (315, 325, 365, 370, 420 nm), and (350, 360, 380, 455, 550 nm), respectively. These findings confirmed the previous hypothesis as most of them corresponded to the maximum emission of hydroxybenzoic acid, flavonols, and flavan-3-ols. These findings have been confirmed by recent research (Tourtoglou et al., 2014; Tzachristas et al., 2021). Specifically, analysis by LC-MS reported the most abundant molecules among the above chemical groups to be gallic acid followed by protocatechuic acid and gentisic acid (Tzachristas et al., 2021). In addition, catechin and epicatechin were found in significant abundance (Tzachristas et al., 2021).
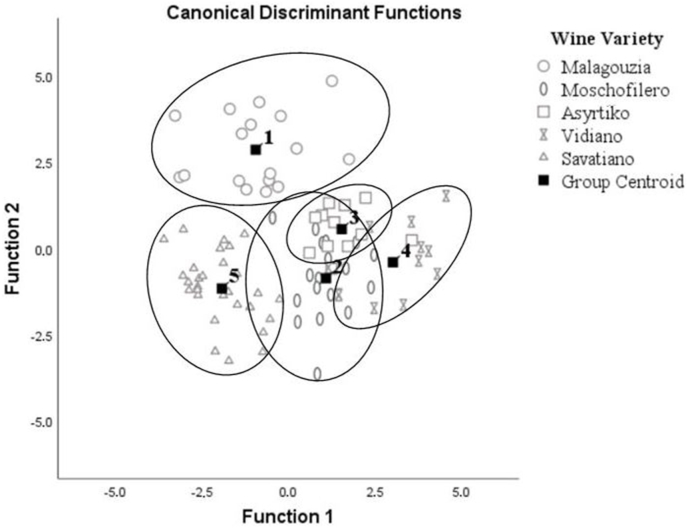
The chemometric model with excitation λex at 310 nm was found to be the more effective with a calibration score of 95.5%. Internal and external validation scores (91.0%; 86.3%) reinforced the robustness of the developed models, as the difference between their percentage sets was minimal. Detailed results for λex at 310 nm are shown in Table 2 and group centroids are also depicted in Fig. 7. This differentiation can be attributed mainly to hydroxycinnamic acids such as caffeic, caftaric, ferulic, sinapic, and p-coumaric acid (Martin et al., 2017), and to a lesser degree to stilbene-like compounds (t-resveratrol, t-piceid, and t-astrigin) (Airado-Rodríguez et al., 2011; Airado-Rodŕiguez et al., 2009; Rodríguez-Delgado et al., 2001). This hypothesis is amplified, since stilbenes are usually found in lower abundance in white wines because of the removal of grape seed and skins during white vinification (Sun et al., 2006). However, even in this case, gentisic acid correlated satisfactorily, and there were also small contributions from hydroxybenzoic acid, flavonoids, and flavan-3-ols, too. Further, the stepwise algorithm pointed out six standardized canonical discriminant function coefficients (350, 395, 400, 450, 545, and 575 nm) which affected the developed model. It is important to mention that it is quite difficult to attribute these coefficients to specific molecules. Despite the presence of several fluorophore compounds in this spectra region, the development of robust models remained unaffected by overlaps. It may be concluded hypothetically that the fluorescence spectrum at λex = 310 nm constitute a multicomponent region (“fingerprint”) which provides relevant information for autochthonous Greek white wines differentiation.
Table 2.
Discrimination results based on fluorescence at λex = 310 nm.
| Predicted Group Membership |
||||||||
|---|---|---|---|---|---|---|---|---|
| Wine Variety | Malagouzia | Moschofilero | Asyrtiko | Vidiano | Savatiano | Total | ||
| Classification | Count | Malagouzia | 17 | 0 | 0 | 0 | 0 | 17 |
| Moschofilero | 0 | 16 | 2 | 0 | 0 | 18 | ||
| Asyrtiko | 0 | 0 | 12 | 0 | 0 | 12 | ||
| Vidiano | 0 | 1 | 1 | 10 | 0 | 12 | ||
| Savatiano | 0 | 0 | 0 | 0 | 30 | 30 | ||
| % | Malagouzia | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Moschofilero | 0.0 | 88.9 | 11.1 | 0.0 | 0.0 | 100.0 | ||
| Asyrtiko | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | ||
| Vidiano | 0.0 | 8.3 | 8.3 | 83.3 | 0.0 | 100.0 | ||
| Savatiano | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | ||
| Internal validation | Count | Malagouzia | 15 | 0 | 1 | 0 | 1 | 17 |
| Moschofilero | 0 | 15 | 3 | 0 | 0 | 18 | ||
| Asyrtiko | 0 | 0 | 12 | 0 | 0 | 12 | ||
| Vidiano | 0 | 2 | 1 | 9 | 0 | 12 | ||
| Savatiano | 0 | 0 | 0 | 0 | 30 | 30 | ||
| % | Malagouzia | 88.2 | 0.0 | 5.9 | 0.0 | 5.9 | 100.0 | |
| Moschofilero | 0.0 | 83.3 | 16.7 | 0.0 | 0.0 | 100.0 | ||
| Asyrtiko | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | ||
| Vidiano | 0.0 | 16.7 | 8.3 | 75.0 | 0.0 | 100.0 | ||
| Savatiano | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | ||
Fig. 7.
Discrimination results based on λex = 310 nm.
4. Conclusions
The chemometric models were developed, to employ fluorescence data at excitation λex 280, 295, and 310 nm, for the purpose of distinguish between five commercial endogenic Greek white wines (Malagouzia, Moschofilero, Asyrtiko, Vidiano, and Savatiano). The application of the stepwise algorithm, which has not been previously applied, in order to discriminate between white wine samples, was performed successfully, achieving a calibration accuracy of 79.8% for λex 280 and 295 nm and 95.5% for λex 310 nm. The models were validated by internal (cross-validation) validation with a rate of 74.2%, 73.0%, and 91.0% for each λex (280; 295, and 310 nm), respectively. Also, external validation (test set) results were similar (74.5%, 73.0%, and 86.3%). The results of this study confirmed that fluorophore phenolic molecules (hydroxycinnamic acids, stilbene-like compounds, hydroxybenzoic acid, flavonoids, and flavan-3-ols) were significantly related to the white wine varieties. Consequently, the novel methodology which was developed based on the simple, non-time-consuming, non-destructive, environmentally friendly fluorescence spectroscopy can be applied for the authentication of autochthonous Greek white wines, thereby protecting consumers from fraudulent practices.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Data availability statement
Not applicable.
CRediT authorship contribution statement
Marinos Xagoraris: Conceptualization, Methodology, Software, Validation, Formal analysis, Data curation, Writing – original draft. Panagiota-Kyriaki Revelou: Formal analysis, Data curation, Visualization, Writing – review & editing. Nikos Arvanitis: Investigation, Data curation. Marianthi Basalekou: Resources, Visualization, Writing – review & editing. Christos S. Pappas: Supervision, Writing – review & editing. Petros A. Tarantilis: Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2021.11.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Airado-Rodríguez D., Durán-Merás I., Galeano-Díaz T., Wold J.P. Front-face fluorescence spectroscopy: a new tool for control in the wine industry. J. Food Compos. Anal. 2011;24(2):257–264. doi: 10.1016/j.jfca.2010.10.005. [DOI] [Google Scholar]
- Airado-Rodŕiguez D., Galeano-&Dacuteiaz T., Durán-Merás I., Wold J.P. Usefulness of fluorescence excitation-emission matrices in combination with parafac, as fingerprints of red wines. J. Agric. Food Chem. 2009;57(5):1711–1720. doi: 10.1021/jf8033623. [DOI] [PubMed] [Google Scholar]
- Anjos O., Caldeira I., Pedro S.I., Canas S. FT-Raman methodology applied to identify different ageing stages of wine spirits. LWT (Lebensm.-Wiss. & Technol.) 2020;134(110179) doi: 10.1016/j.lwt.2020.110179. [DOI] [Google Scholar]
- Azcarate S.M., De Araújo Gomes A., Alcaraz M.R., Ugulino De Araújo M.C., Camiña J.M., Goicoechea H.C. Modeling excitation-emission fluorescence matrices with pattern recognition algorithms for classification of Argentine white wines according grape variety. Food Chem. 2015;184:214–219. doi: 10.1016/j.foodchem.2015.03.081. [DOI] [PubMed] [Google Scholar]
- Azcarate S.M., Cantarelli M.Á., Pellerano R.G., Marchevsky E.J., Camiña J.M. Classification of argentinean sauvignon blanc wines by UV spectroscopy and chemometric methods. J. Food Sci. 2013;78(3) doi: 10.1111/1750-3841.12060. [DOI] [PubMed] [Google Scholar]
- Basalekou M., Pappas C., Tarantilis P.A., Kallithraka S. Vol. 6. Multidisciplinary digital publishing institute; 2020. Wine authenticity and traceability with the use of FT-IR; pp. 1–13. (In beverages). 2. [DOI] [Google Scholar]
- Basalekou M., Pappas C., Tarantilis P., Kotseridis Y., Kallithraka S. Wine authentication with Fourier Transform Infrared Spectroscopy: a feasibility study on variety, type of barrel wood and ageing time classification. Int. J. Food Sci. Technol. 2017;52(6):1307–1313. doi: 10.1111/ijfs.13424. [DOI] [Google Scholar]
- Chen J.Y., Chen X.W., Lin Y.Y., Yen G.C., Lin J.A. Authentication of dark brown sugars from different processing using three-dimensional fluorescence spectroscopy. LWT (Lebensm.-Wiss. & Technol.) 2021;150(111959) doi: 10.1016/j.lwt.2021.111959. [DOI] [Google Scholar]
- Dufour É., Letort A., Laguet A., Lebecque A., Serra J.N. Investigation of variety, typicality and vintage of French and German wines using front-face fluorescence spectroscopy. Anal. Chim. Acta. 2006;563(1–2 SPEC. ISS.):292–299. doi: 10.1016/j.aca.2005.11.005. [DOI] [Google Scholar]
- Hu X.Z., Liu S.Q., Li X.H., Wang C.X., Ni X.L., Liu X., Wang Y., Liu Y., Xu C.H. Geographical origin traceability of Cabernet Sauvignon wines based on Infrared fingerprint technology combined with chemometrics. Sci. Rep. 2019;9(1):1–9. doi: 10.1038/s41598-019-44521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiloglu S. Authenticity and traceability in beverages. Food Chem. 2019;277:12–24. doi: 10.1016/j.foodchem.2018.10.091. [DOI] [PubMed] [Google Scholar]
- Magdas D.A., Cozar B.I., Feher I., Guyon F., Dehelean A., Cinta Pinzaru S. Testing the limits of FT-Raman spectroscopy for wine authentication: cultivar, geographical origin, vintage and terroir effect influence. Sci. Rep. 2019;9(1):1–8. doi: 10.1038/s41598-019-56467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdas D.A., Pirnau A., Feher I., Guyon F., Cozar B.I. Alternative approach of applying 1H NMR in conjunction with chemometrics for wine classification. LWT (Lebensm.-Wiss. & Technol.) 2019;109:422–428. doi: 10.1016/j.lwt.2019.04.054. [DOI] [Google Scholar]
- Martin C., Bruneel J.L., Castet F., Fritsch A., Teissedre P.L., Jourdes M., Guillaume F. Spectroscopic and theoretical investigations of phenolic acids in white wines. Food Chem. 2017;221:568–575. doi: 10.1016/j.foodchem.2016.11.137. [DOI] [PubMed] [Google Scholar]
- Mascellani A., Hoca G., Babisz M., Krska P., Kloucek P., Havlik J. 1H NMR chemometric models for classification of Czech wine type and variety. Food Chem. 2021;339 doi: 10.1016/j.foodchem.2020.127852. [DOI] [PubMed] [Google Scholar]
- Medel-Marabolí M., López-Solís R., Valenzuela-Prieto D., Vargas-Silva S., Obreque-Slier E. Limited relationship between temporality of sensory perception and phenolic composition of red wines. LWT (Lebensm.-Wiss. & Technol.) 2021;142(111028) doi: 10.1016/j.lwt.2021.111028. [DOI] [Google Scholar]
- Platform O.C., Ciberobn J.S. 2020. https://www.europarl.europa.eu/cmsdata/214558/CEEV_Sanchez%20Recarte_Covid%20ISR%20summary%2020201026_v2.pdf Monday, 26 OCTOBER 2020. October, 26–29.
- Popîrdă A., Luchian C.E., Cotea V.V., Colibaba L.C., Scutarașu E.C., Toader A.M. Vol. 11. Multidisciplinary Digital Publishing Institute; 2021. A review of representative methods used in wine authentication; pp. 1–20. (In Agriculture (Switzerland)). 3. [DOI] [Google Scholar]
- Ranaweera R.K.R., Capone D.L., Bastian S.E.P., Cozzolino D., Jeffery D.W. A review of wine authentication using spectroscopic approaches in combination with chemometrics. Molecules. 2021;26(14):4334. doi: 10.3390/molecules26144334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaweera R.K.R., Gilmore A.M., Capone D.L., Bastian S.E.P., Jeffery D.W. Spectrofluorometric analysis combined with machine learning for geographical and varietal authentication, and prediction of phenolic compound concentrations in red wine. Food Chem. 2021;361(130149) doi: 10.1016/j.foodchem.2021.130149. [DOI] [PubMed] [Google Scholar]
- Ranaweera R.K.R., Gilmore A.M., Capone D.L., Bastian S.E.P., Jeffery D.W. Authentication of the geographical origin of Australian Cabernet Sauvignon wines using spectrofluorometric and multi-element analyses with multivariate statistical modelling. Food Chem. 2021;335 doi: 10.1016/j.foodchem.2020.127592. [DOI] [PubMed] [Google Scholar]
- Ríos-Reina R., Elcoroaristizabal S., Ocaña-González J.A., García-González D.L., Amigo J.M., Callejón R.M. Characterization and authentication of Spanish PDO wine vinegars using multidimensional fluorescence and chemometrics. Food Chem. 2017;230:108–116. doi: 10.1016/j.foodchem.2017.02.118. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Delgado M.A., Malovaná S., Pérez J.P., Borges T., García Montelongo F.J. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. A. 2001;912(2):249–257. doi: 10.1016/S0021-9673(01)00598-2. [DOI] [PubMed] [Google Scholar]
- Sádecká J., Jakubíková M. Varietal classification of white wines by fluorescence spectroscopy. J. Food Sci. Technol. 2020;57(7):2545–2553. doi: 10.1007/s13197-020-04291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schueuermann C., Silcock P., Bremer P. Front-face fluorescence spectroscopy in combination with parallel factor analysis for profiling of clonal and vineyard site differences in commercially produced Pinot Noir grape juices and wines. J. Food Compos. Anal. 2018;66:30–38. doi: 10.1016/j.jfca.2017.11.005. [DOI] [Google Scholar]
- Strasburg G.M., Ludescher R.D. Trends in Food Science and Technology. Vol. 6. Elsevier; 1995. Theory and applications of fluorescence spectroscopy in food research; pp. 69–75. 3. [Google Scholar]
- Suciu R.C., Zarbo L., Guyon F., Magdas D.A. Application of fluorescence spectroscopy using classical right angle technique in white wines classification. Sci. Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-54697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Ribes A.M., Leandro M.C., Belchior A.P., Spranger M.I. Stilbenes: quantitative extraction from grape skins, contribution of grape solids to wine and variation during wine maturation. Anal. Chim. Acta. 2006;563(1–2 SPEC. ISS.):382–390. doi: 10.1016/j.aca.2005.12.002. [DOI] [Google Scholar]
- Tourtoglou C., Nenadis N., Paraskevopoulou A. Phenolic composition and radical scavenging activity of commercial Greek white wines from Vitis vinifera L. cv. Malagousia. J. Food Compos. Anal. 2014;33(2):166–174. doi: 10.1016/j.jfca.2013.12.009. [DOI] [Google Scholar]
- Tufariello M., Pati S., D'Amico L., Bleve G., Losito I., Grieco F. Quantitative issues related to the headspace-SPME-GC/MS analysis of volatile compounds in wines: the case of Maresco sparkling wine. LWT (Lebensm.-Wiss. & Technol.) 2019;108:268–276. doi: 10.1016/j.lwt.2019.03.063. [DOI] [Google Scholar]
- Tzachristas A., Dasenaki M., Aalizadeh R., Thomaidis N.S., Proestos C. LC-MS based metabolomics for the authentication of selected Greek white wines. Microchem. J. 2021;169(106543) doi: 10.1016/j.microc.2021.106543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin L., Barroso L.P., Barbosa R.M., de Paulo G.A., Castro I.A. Chemical typicality of South American red wines classified according to their volatile and phenolic compounds using multivariate analysis. Food Chem. 2020;302 doi: 10.1016/j.foodchem.2019.125340. [DOI] [PubMed] [Google Scholar]
- Wine market revenue worldwide 2012-2025, Statista. https://www.statista.com/statistics/922403/global-wine-market-size/ (n.d.). Retrieved August 21, 2021, from.
- Ziółkowska A., Wąsowicz E., Jeleń H.H. Differentiation of wines according to grape variety and geographical origin based on volatiles profiling using SPME-MS and SPME-GC/MS methods. Food Chem. 2016;213:714–720. doi: 10.1016/j.foodchem.2016.06.120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.