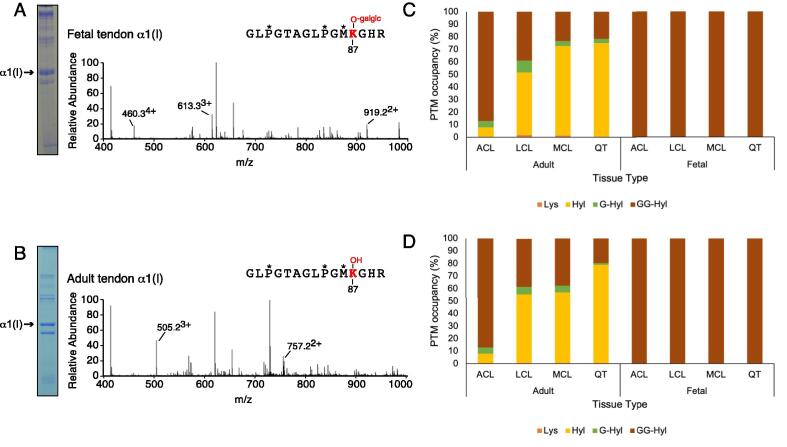
Fig. 3.
Post-translational variances in linear cross-linking α1(I)K87 and α2(I)87 from type I collagens. (A, B) LC-MS profiles of in-gel trypsin digests of the collagen α1(I) chain from fetal and adult bovine tendon. (A) Fetal bovine tendon shows complete glycosylation of α1(I)K87 (613.33+). (B) The α1(I)K87 residue from adult bovine tendon is hydroxylated but not subsequently glycosylated (505.23+). The trypsin-digested peptide is shown with P* indicating 4Hyp, M* indicating oxidized methionine. Summary of modifications on lysine 87 from (C) α1(I) and (D) α2(I) chains of type I collagen. Lysine modifications include unmodified lysine (Lys), hydroxylysine (Hyl), galactosyl-hydroxylysine (G-Hyl) and glucosylgalactosyl-hydroxylysine (GG-Hyl). The percentages were determined based on the ratios between the ion-current yields of each post-translational variant as previously described. See Table 2 for more details.